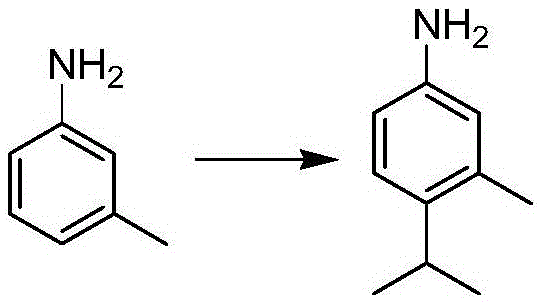
Preparation method of 3-methyl-4-isopropylaniline
A technology of isopropylaniline and toluidine, which is applied in the field of preparation of organic intermediates, can solve the problems of low production capacity, unsuitable for industrial production, and difficult availability of raw materials, etc., and achieves low pollution, few by-products, and high reaction efficiency. The effect of mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Add 1900.0g of sulfuric acid solution (95wt%) into the four-necked flask, stir and cool down to between 0 and 10°C, add 214.3g of m-toluidine dropwise, keep the temperature during the dropping process between 0 and 10°C, and control the drop for 2 hours. After the addition is completed, continue to stir for 30 minutes, and use it as component 1 for later use. Add 300.0 g of 2-chloropropane in another flask as component 2, and use a peristaltic pump to mix component 1 and component 2 at a ratio of 7:1. The flow rate (feed rate weight ratio) is simultaneously input into the T-type jet mixer and mixed rapidly at 20°C, and then pressed into the zirconia quartz reaction tube (microreactor) equipped with 6.0g of sulfuric acid, at 80-85 ℃ reaction to obtain the reaction solution of the alkylation reaction; the diameter of the quartz reaction tube is 2.5mm, the length of the tube is 2.0m, the residence time is 28s, and the particle size of the zirconia loaded with sulfuric acid ...
Embodiment 2
[0028] Add 2300.0g of sulfuric acid solution (80wt%) into the four-necked flask, stir and cool down to between 0 and 10°C, add 214.3g of m-toluidine dropwise, keep the temperature during the dropping process between 0 and 10°C, and control the drop for 2 hours. After the addition is completed, continue to stir for 30 minutes, and use it as component 1 for later use. Add 250g of isopropanol to another flask as component 2, and use a peristaltic pump to simultaneously mix components 1 and 2 at a flow rate of 10:1. Input it into a T-type jet mixer and mix rapidly at 30°C, then press it into a zirconia quartz reaction tube containing 6.0g of sulfuric acid, and react at 85-90°C to obtain the reaction solution of the alkylation reaction; the quartz reaction tube The pipe diameter is 5mm, the pipe length is 5m, the residence time is 40s, and the particle size of the zirconia loaded with sulfuric acid is 1.0mm; a 3L four-necked bottle is used to receive the reaction solution.
[0029]...
Embodiment 3
[0032] Add 2000.0g of sulfuric acid solution (85wt%) into the four-necked flask, stir and cool down to between 0 and 10°C, add 214.3g of m-toluidine dropwise, keep the temperature during the dropping process between 0 and 10°C, and control the drop for 2 hours. After the addition is completed, continue to stir for 30 minutes, and use it as component 1 for later use. In another flask, add 220 g of isopropanol as component 2, and use a peristaltic pump to mix component 1 and component 2 at a flow rate of 9:1 ( Feed rate (weight ratio) is simultaneously input into a T-type jet mixer for rapid mixing, and the mixing temperature is controlled at 25°C; then pressed into a zirconia quartz reaction tube containing 6.0g of sulfuric acid, and reacted at 70-75°C to obtain alkane The reaction solution of the radicalization reaction; the pipe diameter of the microreactor is 2.5mm, the pipe length is 2.0m, the residence time is 25s, and the particle size of the zirconia loaded with sulfuric ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com