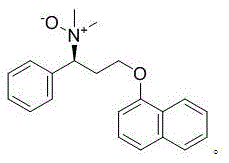
Preparation method of dapoxetine N-oxide
A technology of urea hydrogen peroxide and naphthyloxy, which is used in the fields of medicine and chemical industry, and can solve the problems of N-oxidation-dapoxetine synthesis and methods that have not been reported.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 Preparation of N-oxidation-Dapoxetine
[0039] Add 50ml of dichloromethane and 10.0g of (S)-N,N-dimethyl-(α)-[2-(1-naphthyloxy)ethyl]benzylamine to the reaction flask, stir and cool to -5°C , Slowly add a dichloromethane solution of urea hydrogen peroxide (6.2g) dropwise, keeping the internal temperature <-5°C, and TLC detects the end of the reaction. After the reaction is complete, add sodium bicarbonate aqueous solution, stir and separate the liquids. The organic phase was washed once with saturated brine, dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated to dryness under reduced pressure to obtain a brown residue, which was purified by column chromatography and concentrated under reduced pressure to dryness to obtain a brown solid N-oxide-dapoxetine 2.2g, the molar yield is 20.91%.
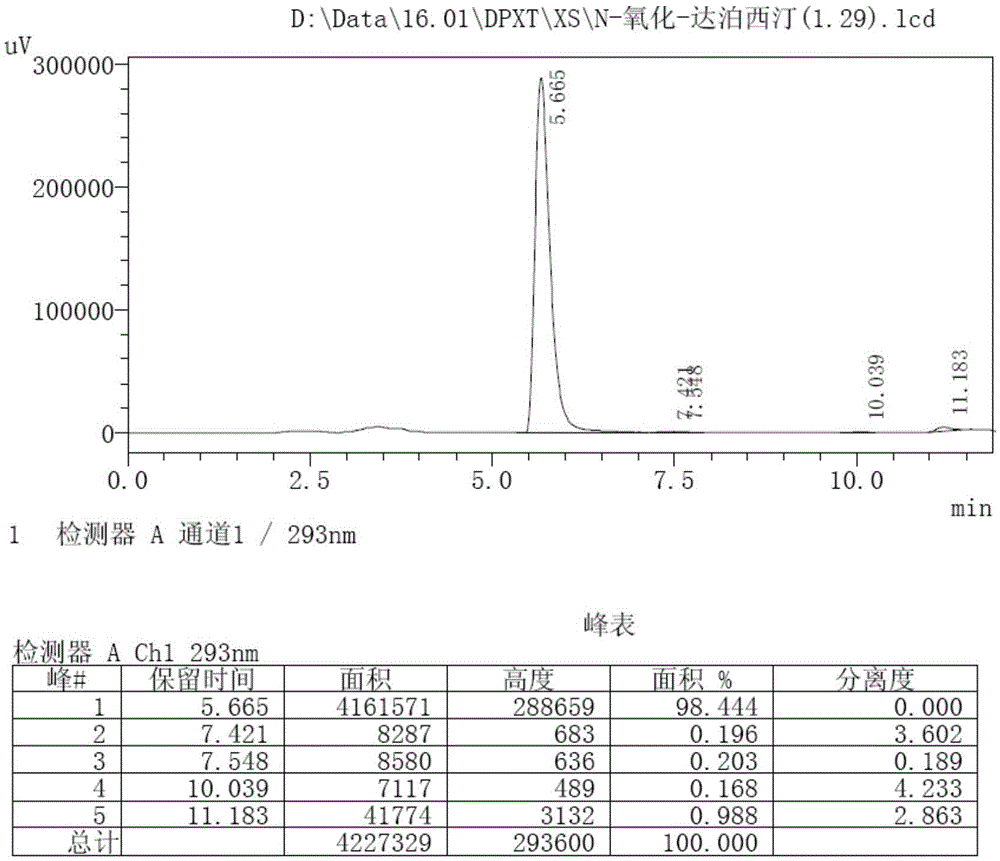
[0040] According to the HPLC detection conditions of related substances under the item of Dapoxetine raw materials, the detection spectrum of N-oxid...
Embodiment 2
[0041] Example 2 Preparation of N-oxidation-Dapoxetine
[0042] Add 50ml of dichloromethane and 10.0g of (S)-N,N-dimethyl-(α)-[2-(1-naphthyloxy)ethyl]benzylamine to the reaction flask, stir and cool to -5°C , Slowly add a dichloromethane solution of urea hydrogen peroxide (9.3g) dropwise, keep the internal temperature <-5°C, and detect the end of the reaction by TLC. After the reaction is complete, add sodium bicarbonate aqueous solution, stir and separate the liquids. The organic phase was washed once with saturated brine, dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated to dryness under reduced pressure to obtain a brown residue, which was purified by column chromatography and concentrated under reduced pressure to dryness to obtain a brown solid N-oxide-dapoxetine 2.6g, the molar yield is 24.71%.
Embodiment 3
[0043] Example 3 Preparation of N-oxidation-Dapoxetine
[0044] Add 50ml of chloroform and 10.0g of (S)-N,N-dimethyl-(α)-[2-(1-naphthyloxy)ethyl]benzylamine to the reaction flask, stir and cool to -5°C , Slowly add a chloroform solution of urea hydrogen peroxide (3.1g) dropwise, keep the internal temperature <-5°C, and detect the end of the reaction by TLC. After the reaction is complete, add sodium bicarbonate aqueous solution, stir and separate the liquids. The organic phase was washed once with saturated brine, dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated to dryness under reduced pressure to obtain a brown residue, which was purified by column chromatography, and concentrated under reduced pressure to dryness to obtain a brown solid N-oxide-dapoxetine 1.8g, the molar yield is 17.11%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com