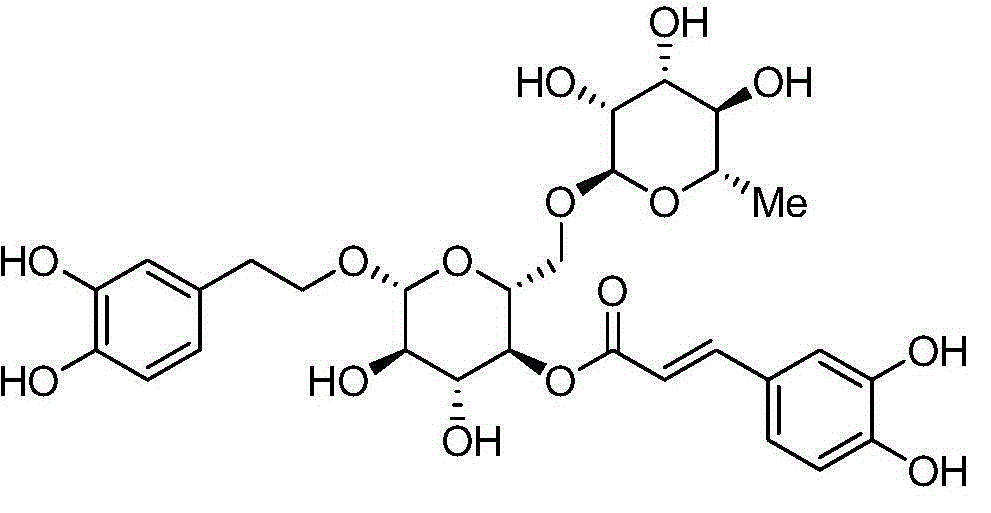
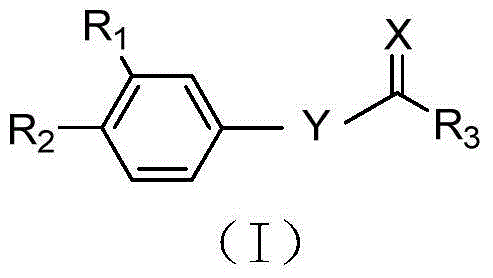
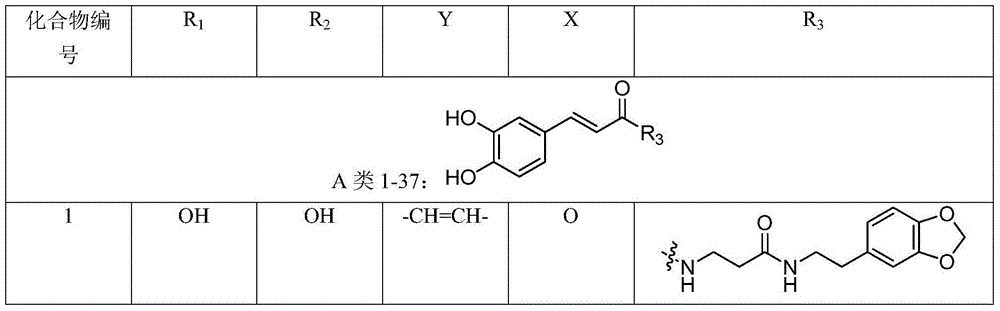
Synergist for antifungal drugs, and preparation and application thereof
A technology of antifungal drugs and synergists, applied in the field of medicine, can solve the problems of transformation, no structure of forsythiaside, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Example 1: (E)-N-(3-(2-(benzo[d][1,3]dioxol-5-yl)ethylamino)-3-oxopropyl) - Preparation of 3-(3,4-dihydroxyphenyl)acrylamide (1)
[0084] step one: preparation of
[0085] Weigh the Boc-protected amino acid (10mmol), HOBt (1.35g, 10mmol) and DCC (2.06g, 10mmol) in a 100ml eggplant-shaped bottle, add THF (30ml) to dissolve it, weigh piperonyl ethylamine (1.65g, 10ml ) was dissolved in 10mlTHF and slowly dropped into the above-mentioned reaction solution. After the dropwise addition was completed, the reaction was stirred overnight at room temperature, and the reaction was detected by TLC. After the reaction was completed, the insoluble matter was removed by filtration. dissolved, washed three times with saturated aqueous sodium bicarbonate solution, three times with anhydrous sodium chloride, and dried over anhydrous sodium sulfate. Silica gel column chromatography gives product 1: tert-butyl-3-(2-(benzo[d][1,3]dioxol-5-yl)ethylamino-3-oxopropylaminomethyl esters. ...
Embodiment 2
[0094] Example 2: (E)-(2-(benzo[d][1,3]dioxol-5-yl)ethyl)-(3-(3-dihydroxyphenyl)acryloyl )-4-formamide piperidine (2) preparation
[0095] step one: preparation of
[0096] Weigh the Boc-protected amino acid (10mmol), HOBt (1.35g, 10mmol) and DCC (2.06g, 10mmol) in a 100ml eggplant-shaped bottle, add THF (30ml) to dissolve it, weigh piperonyl ethylamine (1.65g, 10ml ) was dissolved in 10mlTHF and slowly dropped into the above-mentioned reaction solution. After the dropwise addition was completed, the reaction was stirred overnight at room temperature, and the reaction was detected by TLC. After the reaction was completed, the insoluble matter was removed by filtration. dissolved, washed three times with saturated aqueous sodium bicarbonate solution, three times with anhydrous sodium chloride, and dried over anhydrous sodium sulfate. Silica gel column chromatography obtains product 3: tert-butyl-4-(2-(benzo[d][1,3]dioxol-5-yl) ethylcarbamoyl) tert-butylpiperidine tert-Buty...
Embodiment 3
[0105] Example 3: (E)-N-(2-(benzo[d][1,3]dioxol-5-yl)ethyl)-(3-(3-dihydroxyphenyl) Preparation of Acryloylpyrrolidine-2-Carboxamide (3)
[0106] step one: preparation of
[0107] Weigh the Boc-protected amino acid (10mmol), HOBt (1.35g, 10mmol) and DCC (2.06g, 10mmol) in a 100ml eggplant-shaped bottle, add THF (30ml) to dissolve it, weigh piperonyl ethylamine (1.65g, 10ml ) was dissolved in 10mlTHF and slowly dropped into the above-mentioned reaction solution. After the dropwise addition was completed, the reaction was stirred overnight at room temperature, and the reaction was detected by TLC. After the reaction was completed, the insoluble matter was removed by filtration. dissolved, washed three times with saturated aqueous sodium bicarbonate solution, three times with anhydrous sodium chloride, and dried over anhydrous sodium sulfate. Silica gel column chromatography gave product 5: tert-butyl-2-(2-(benzo[d][1,3]dioxol-5-yl)ethylcarbamoyl)pyrrolidine-1- tert-butyl for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com