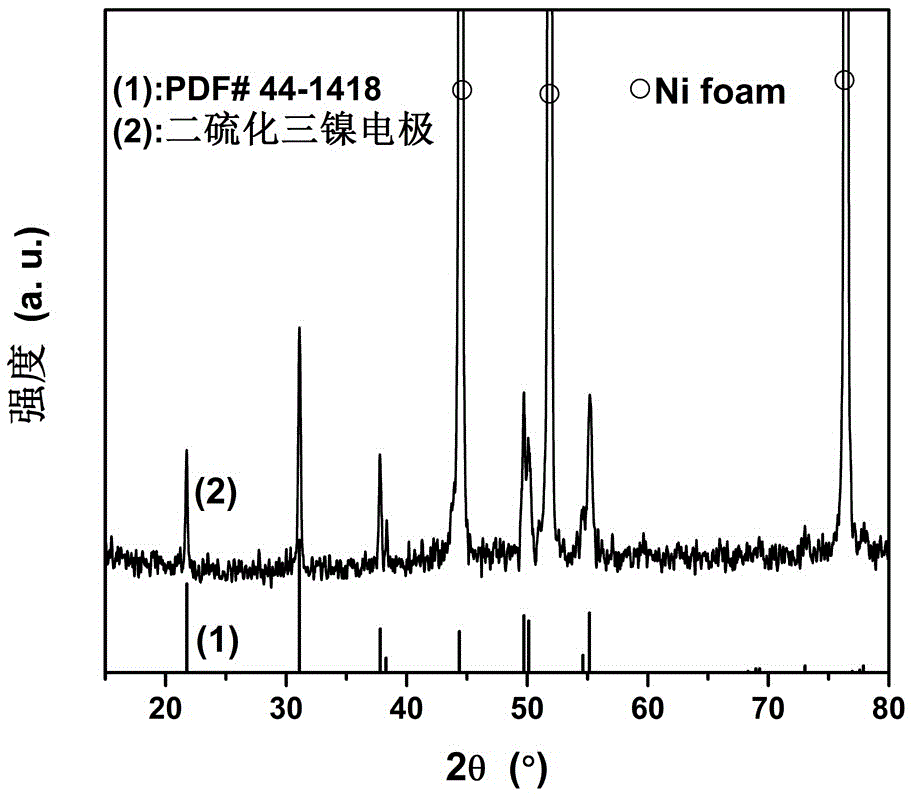
Application of trinickel disulfide electrode material to electro-catalytic oxidation of hydrazine hydrate
A technology of nickel disulfide and electrocatalytic oxidation, which is used in chemical analysis, battery electrodes, circuits, etc. using catalysis, and can solve problems such as unseen patent applications.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
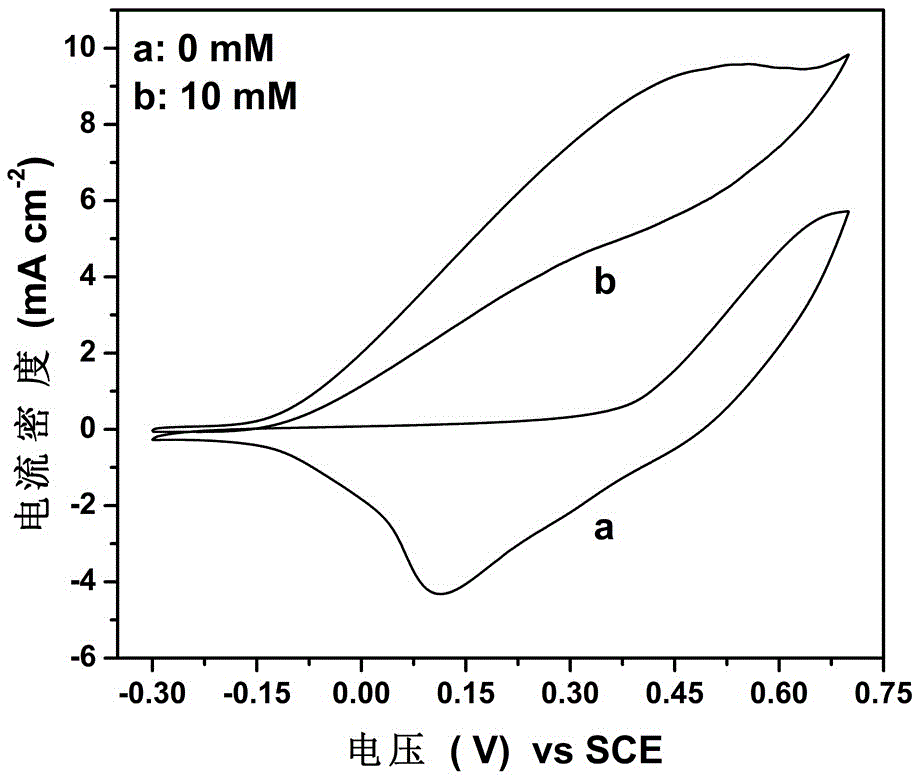
[0028] Cyclic voltammetry curves obtained at a scan rate of 50 mV / s in 0 and 10 mM hydrazine hydrate solutions using an electrode based on nickel disulfide, as figure 2 . After adding hydrazine hydrate, the curve appears about the electrocatalytic oxidation peak of hydrazine hydrate at 0.5V, and the peak current density is as high as 9.64mAcm –2 . At the same time, the curve rises rapidly at –0.15V, indicating a lower initial voltage. It is shown that nickel trisulfide has a high ability to electrocatalyze the oxidation of hydrazine hydrate, including high current density and low onset potential, and can be used as an anode material for hydrazine hydrate fuel cells.
Embodiment 2
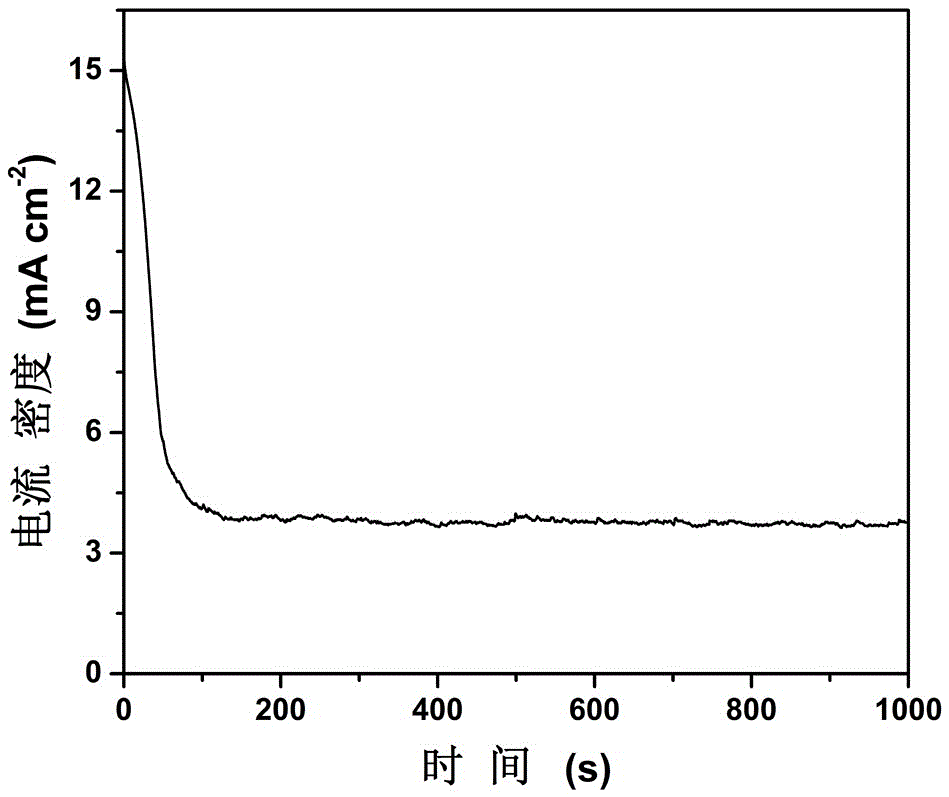
[0030] Using an electrode based on nickel disulfide, test its chronoamperometry curve to 10mM hydrazine hydrate at a working voltage of 0.5V, such as image 3 . After the electrode reaches its diffusion equilibrium in 100s, it maintains a stable current density in the next 900s, indicating that nickel disulfide has good durability for the electrocatalytic oxidation of hydrazine hydrate and can be used as an anode material for hydrazine hydrate fuel cells.
Embodiment 3
[0032] Using an electrode based on nickel disulfide, at a working voltage of 0.5V, test its response to different concentrations (1, 10, 20, 30, 40, 50, 60, 70, 80, 90, 100, 200, 300, 400 , 500μM) sensitive properties of hydrazine hydrate, such as Figure 4 , 5 shown. The response time of the electrode to 1μM hydrazine hydrate is only 4.8s, and within the measured concentration range, the response value can be well maintained after reaching equilibrium; at the same time, it has good linearity in the test range of 1 to 300μM, and the calculated detection limit As low as 0.045μM. It shows that nickel trisulfide has good dynamic response characteristics, high sensitivity and good linearity to hydrazine hydrate, and it can be applied to the electrode material of hydrazine hydrate electrochemical sensor.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com