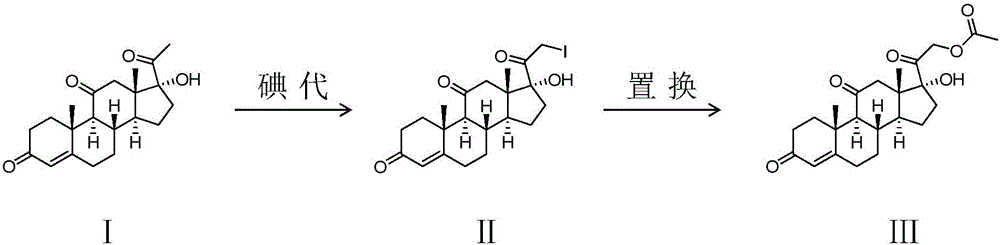
Preparing method of cortisone acetate
A technology of cortisone acetate and iodine solution, applied in the field of chemistry, can solve the problems of easy sublimation of iodine monoplex, large environmental pollution and high cost, and achieve the effects of low cost, reduced environmental pollution and reduced iodine consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Step 1, the preparation of bromine-iodine solution
[0021] Put 200L of methanol and 19.5kg of anhydrous calcium chloride into the bromine-iodine solution preparation tank. When the temperature is less than 25°C, put 57.5kg of iodine solution into the preparation tank and stir for more than 1.0 hours; vacuum pulls in 33.4kg of bromine, After continuing to stir and fully dissolve, pull in the bromine-iodine solution and add it dropwise to the high-level tank, and set aside;
[0022] Step 2, iodination reaction
[0023] Add 19.5kg calcium chloride and 200L methanol in the reaction device, add 650L chloroform, 130kg reactant 17α-hydroxyl-pregn-4-ene-3,11,20-trione (I) after dissolving, control temperature is 10 At about ℃, add 75kg of calcium oxide, continue to cool down to below -3 ℃, slowly add bromine iodine solution, control the temperature not higher than 0 ℃, first slowly and then quickly, about 1-1.5h to finish the drop, after the drop is over -3 Incubate at ~0°C ...
Embodiment 2
[0027] Step 1, the preparation of bromine-iodine solution
[0028] Put 200L of methanol and 19.5kg of anhydrous zinc chloride into the bromine-iodine solution preparation tank. When the temperature is less than 25°C, put 57.5kg of iodine solution into the preparation tank and stir for more than 1.0 hours; vacuum pulls in 33.4kg of bromine , continue to stir and fully dissolve, pull in the bromine-iodine solution and add it dropwise to the high-level tank, and set aside;
[0029] Step 2, iodination reaction
[0030] Add 19.5kg zinc chloride and 200L methanol in the reaction device, add dichloromethane, 130kg reactant 17α-hydroxyl-pregna-4-ene-3,11,20-triketone (I) after dissolving, control temperature is At about 10°C, add 75kg of zinc oxide, continue to cool down to below -3°C, slowly add bromine-iodine solution, control the temperature not higher than 0°C, first slowly and then quickly, about 1-1.5h to finish dripping, after dripping - Incubate at 3-0°C for 3-3.5h, after th...
Embodiment 3
[0034] The reaction steps and conditions are basically the same as in Example 1, except that zinc chloride is used in steps 1 and 2 to replace calcium chloride as a cosolvent.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com