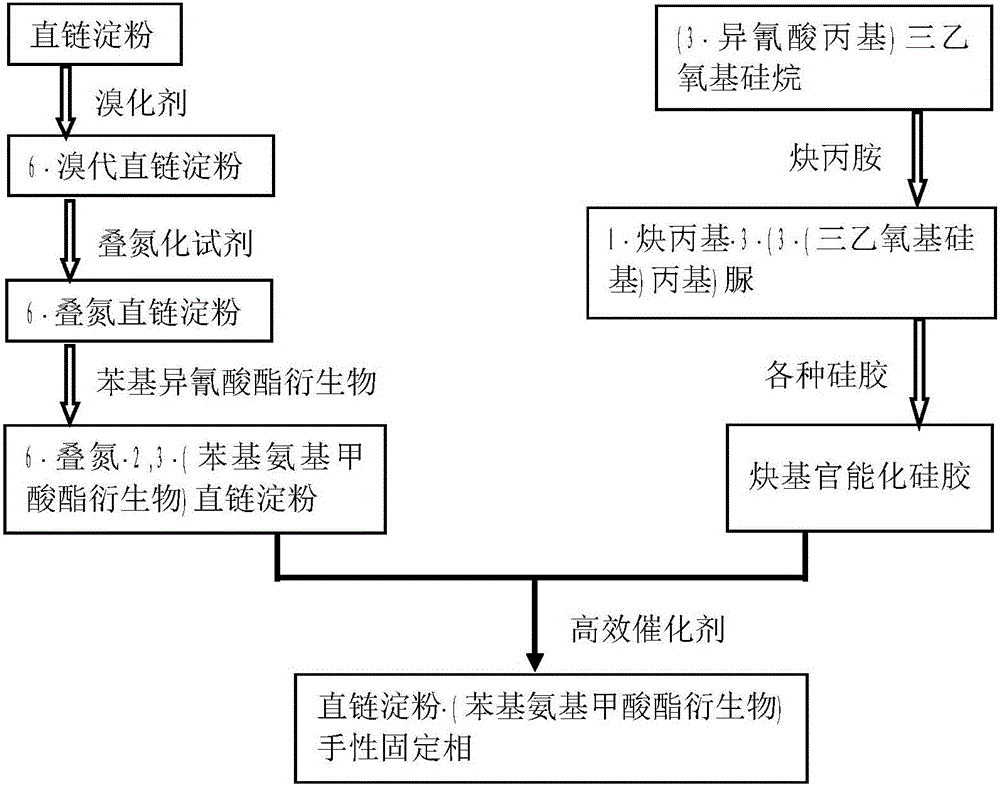
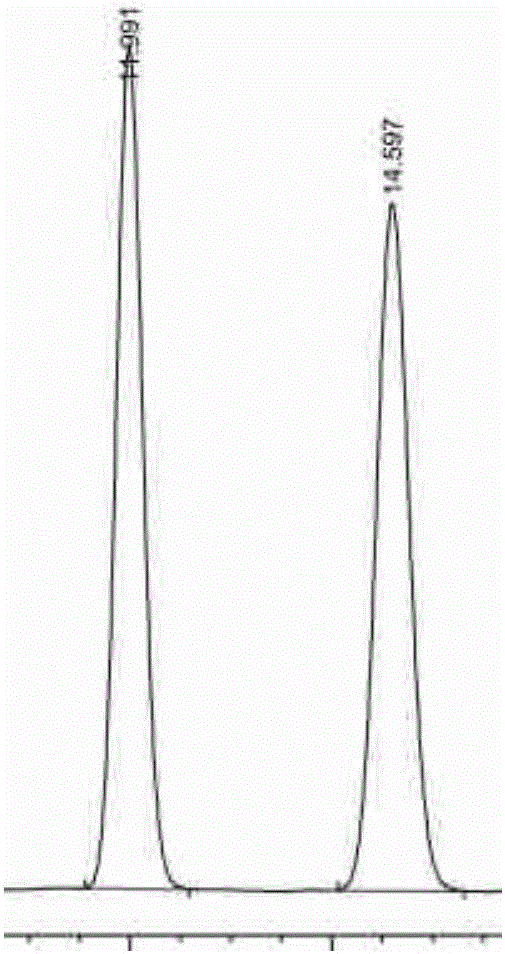
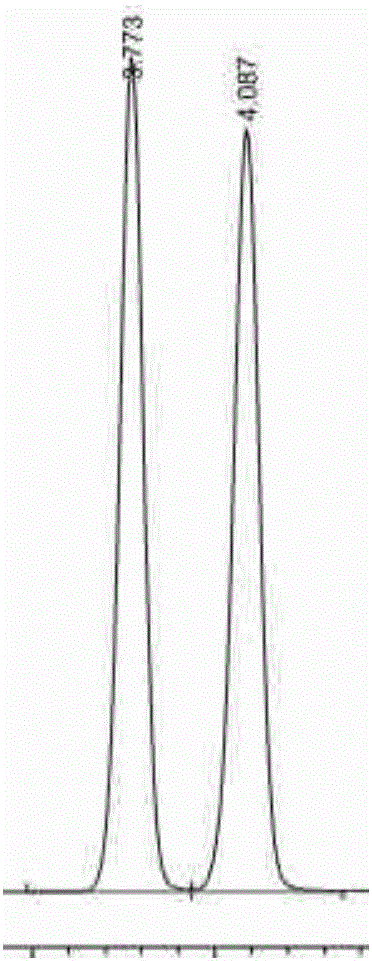
Amylose chiral stationary phase and preparation method thereof
A chiral stationary phase, amylose technology, applied in chemical instruments and methods, other chemical processes, etc., to achieve the effect of stable structure, high selectivity and large amount of bonding
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: Taking silica gel-bonded amylose-(4-chlorophenylcarbamate) chiral stationary phase (CSP1) as an example
[0037] The present embodiment proposes a kind of preparation method of amylose chiral stationary phase, comprises the following steps:
[0038] In the first step, take a 1L double-necked round-bottom flask and then vacuumize it and then blow it with nitrogen gas. Weigh the amylose (10g) after vacuum drying at 100°C and add it to the flask, then add 500mL of anhydrous N,N-dimethyl ethyl Amide (DMA), heated to 80°C and stirred overnight. After cooling to room temperature, under a nitrogen atmosphere, anhydrous lithium chloride (21 g, 0.50 mol) and N-bromosuccinimide (NBS, 5.5 g, 31 mmol) were successively added to the flask, and reacted at room temperature for 12 h. After the reaction finished, the reaction solution was poured into 5L methanol for precipitation, filtered, and vacuum-dried to obtain the product 6-bromoamylose (12.1g, yield 87%); its reactio...
Embodiment 2
[0053] Example 2: Taking silica gel-bonded amylose-(3,5-dimethylphenylcarbamate) chiral stationary phase (CSP2) as an example
[0054] The present embodiment proposes a kind of preparation method of amylose chiral stationary phase, comprises the following steps:
[0055] Step 1: Take a 1L double-necked round-bottom flask and evacuate it first, then ventilate it with nitrogen gas. Weigh the amylose (15g) dried in vacuum at 100°C and add it into the flask, then add 600mL of anhydrous DMA, heat up to 80°C and stir overnight. . After cooling to room temperature, under a nitrogen atmosphere, anhydrous LiCl (47 g, 1.11 mol) and NBS (32.9 g, 0.185 mol) were successively added to the flask, and reacted at room temperature for 12 h. After the reaction, the reaction solution was poured into 8L of methanol for precipitation, filtered, and vacuum-dried to obtain the product 6-bromoamylose.
[0056] In the second step, the first step product 6-bromoamylose (7g) was added to a 1L two-neck...
Embodiment 3
[0063] Example 3: Taking silica gel-bonded amylose-(4-chloro-3-methylphenylcarbamate) chiral stationary phase (CSP3) as an example
[0064] The present embodiment proposes a kind of preparation method of amylose chiral stationary phase, comprises the following steps:
[0065] In the first step, take a 2L double-necked round-bottom flask, first vacuum and then nitrogen, weigh the amylose (21g) that has been vacuum-dried at 100°C and add it to the flask, then add 1.4L of anhydrous DMA, and heat up to 80°C overnight Stir. After cooling to room temperature, under nitrogen atmosphere, anhydrous LiCl (77.1 g, 1.82 mol) and NBS (92.5 g, 0.52 mol) were successively added to the flask, and reacted at room temperature for 12 h. After the reaction, the reaction solution was poured into 25L of methanol for precipitation, filtered, and vacuum-dried to obtain the product 6-bromoamylose.
[0066] In the second step, the first step product 6-bromoamylose (15g) was added to a 2L two-necked f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| separation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com