Novel technical method for reducing nitrobenzene by using iron
A technology of nitrobenzene and electrochemical technology, which is applied in the new technical field of iron reduction of nitrobenzene, can solve problems such as green primary battery reaction of iron reduction of nitrobenzene, and the like, and achieve the effect of avoiding pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
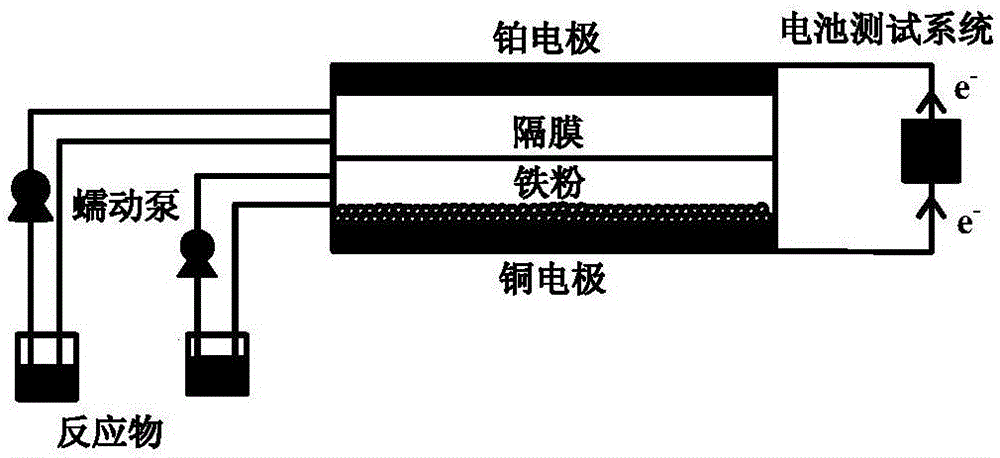
[0028] The cathode is a platinum sheet, the anode is a copper sheet, the anion exchange membrane is a diaphragm, and the catholyte is 0.5molL -1 Nitrobenzene, 3.5molL -1 Ethanol solution of hydrochloric acid, anolyte is iron powder, 0.5molL -1 Aqueous ammonium chloride solution, the reaction temperature is 70°C, the distance between the poles is 1cm, at 10mA / cm 2 current density for the discharge reaction. The maximum power obtained from the battery is 2.0mWcm -2 The cathode product is 26% of aniline, 43% of p-chloroaniline, 8% of o-chloroaniline, 20% of p-ethoxyaniline, and 3% of others; the cathode product of ferrous chloride is 100%.
Embodiment 2
[0030] The cathode is a platinum sheet, the anode is a copper sheet, the anion exchange membrane is a diaphragm, and the catholyte is 1molL -1 Nitrobenzene, 7molL -1 Ethanol solution of hydrochloric acid, anolyte is iron powder, 0.5molL -1 Aqueous ammonium chloride solution, the reaction temperature is 75°C, the distance between the poles is 1cm, at 10mA / cm 2 current density for the discharge reaction. The maximum battery power obtained is 3.2mWcm -2 The cathode product is 30% of aniline, 45% of p-chloroaniline, 9% of o-chloroaniline, 12% of p-ethoxyaniline, and 4% of others; the cathode product of ferrous chloride is 100%.
Embodiment 3
[0032] The cathode is a platinum sheet, the anode is a copper sheet, the anion exchange membrane is a diaphragm, and the catholyte is 2molL -1 Nitrobenzene, 7molL -1 Ethanol solution of hydrochloric acid, anolyte is iron powder, 0.5molL -1 Aqueous ammonium chloride solution, the reaction temperature is 75°C, the distance between the poles is 1cm, at 10mA / cm 2 current density for the discharge reaction. The maximum power obtained from the battery is 4.6mWcm -2 The cathode product is 27% of aniline, 42% of p-chloroaniline, 6% of o-chloroaniline, 14% of p-ethoxyaniline, 6% of azobenzene oxide, and 5% of others; the cathode product of ferrous chloride is 100%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com