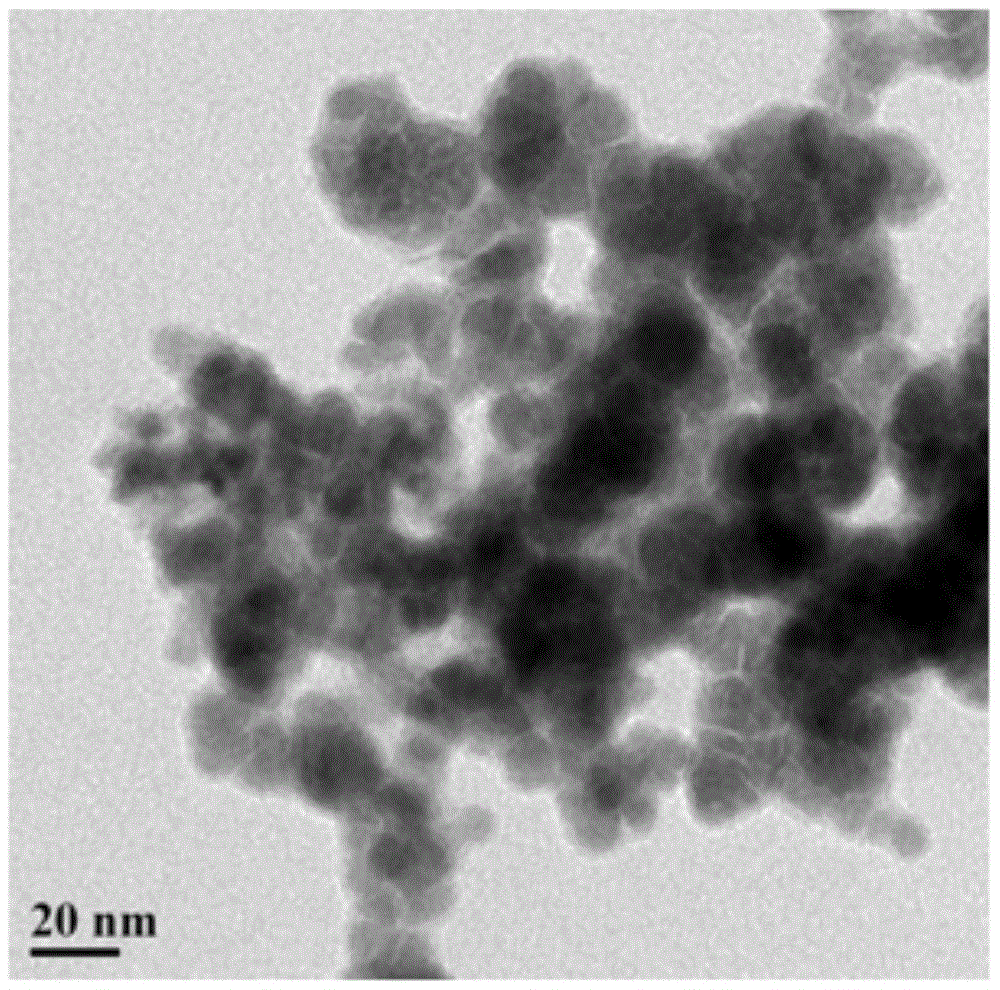
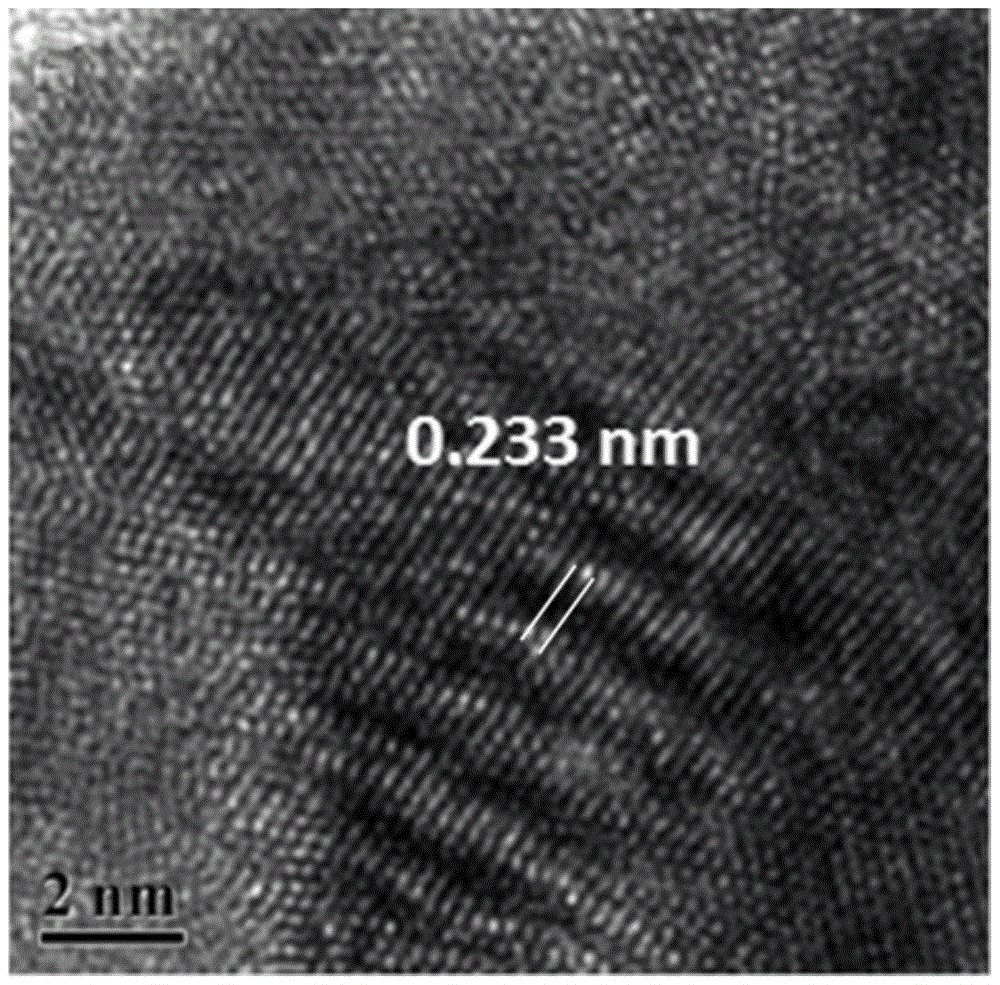
Core-shell gold@ cobalt-boron catalyst for fuel cell
A technology of fuel cells and catalysts, applied in battery electrodes, circuits, electrical components, etc., can solve the problems of cumbersome steps and complicated operation methods, and achieve the effects of simple preparation methods, superior catalytic performance, and good dispersibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The catalyst of this embodiment is a gold-cobalt-boron alloy with a core-shell structure, wherein amorphous cobalt-boron is the shell, and crystalline gold is the core. The preparation method of the catalyst is as follows:
[0029] Step 1. Dissolve 0.01mol cobalt dichloride hexahydrate and 0.001mol chloroauric acid tetrahydrate in deionized water to prepare a 100mL mixed solution; the total concentration of cobalt ions and gold ions in the mixed solution is 0.11mol / L ;
[0030] Step 2, dissolving 0.033mol potassium borohydride in deionized water to prepare a potassium borohydride solution with a concentration of 0.1mol / L, then adding potassium hydroxide to the potassium borohydride solution until the pH of the solution is 11 to obtain boron Potassium hydride-potassium hydroxide mixed solution;
[0031] Step 3: Add the borohydride-hydroxide mixed solution described in Step 2 to the mixed solution described in Step 1 at a rate of 1 mL / min under stirring conditions at a t...
Embodiment 2
[0040] The catalyst of this embodiment is a gold-cobalt-boron alloy with a core-shell structure, wherein amorphous cobalt-boron is the shell, and crystalline gold is the core. The preparation method of the catalyst is as follows:
[0041] Step 1, 0.1mol cobalt dichloride hexahydrate and 0.001mol chloroauric acid tetrahydrate are dissolved in deionized water, and the total concentration of cobalt ion and gold ion is prepared as a mixed solution of 0.1mol / L;
[0042] Step 2, dissolving 0.505mol potassium borohydride in deionized water to prepare a potassium borohydride solution with a concentration of 0.5mol / L, then adding potassium hydroxide to the potassium borohydride solution until the pH of the solution is 12 to obtain boron Potassium hydride-potassium hydroxide mixed solution;
[0043] Step 3: Add the borohydride-hydroxide mixed solution described in Step 2 to the mixed solution described in Step 1 at a rate of 2 mL / min under stirring conditions at a temperature of 10°C, a...
Embodiment 3
[0047] The catalyst of this embodiment is a gold-cobalt-boron alloy with a core-shell structure, wherein amorphous cobalt-boron is the shell, and crystalline gold is the core. The preparation method of the catalyst is as follows:
[0048] Step 1, 0.1mol cobalt dichloride hexahydrate and 0.05mol chloroauric acid tetrahydrate are dissolved in deionized water to prepare a mixed solution whose total concentration of cobalt ion and gold ion is 1mol / L;
[0049] Step 2, dissolving 0.75mol potassium borohydride in deionized water to prepare a potassium borohydride solution with a concentration of 1mol / L, then adding potassium hydroxide to the potassium borohydride solution until the pH value of the solution is 11.5, to obtain borohydride Potassium-potassium hydroxide mixed solution;
[0050] Step 3. Add the borohydride-hydroxide mixed solution described in step 2 to the mixed solution described in step 1 at a rate of 1 mL / min under stirring conditions at a temperature of 15°C, and wai...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Maximum power density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com