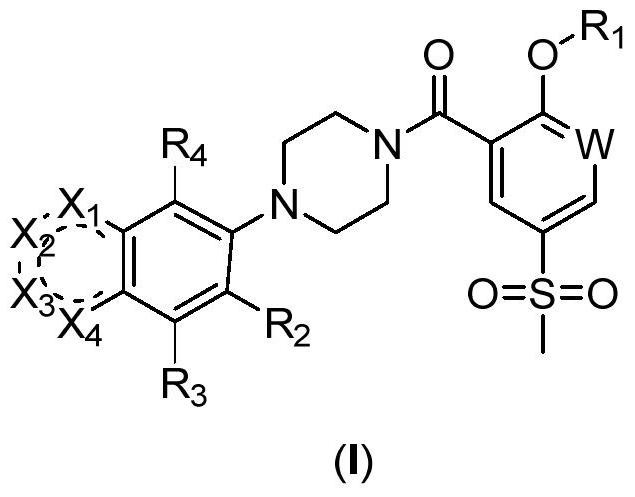
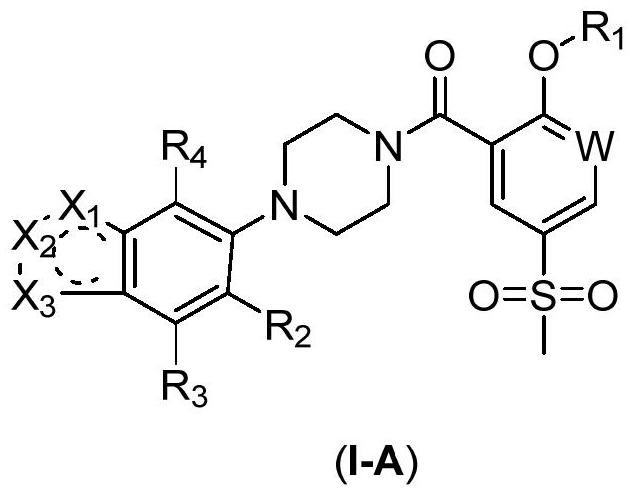
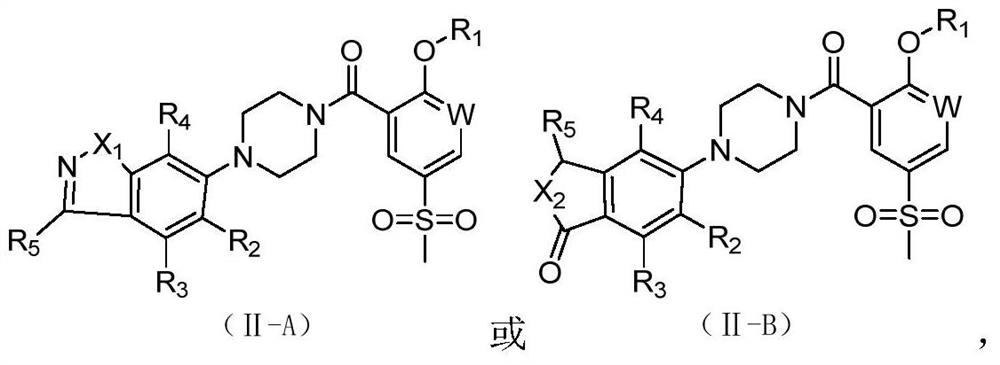
2-Substituted oxy-5-thiamphenicol aryl piperazine amide analogue and its preparation method and use
A technology of aryl piperazine amides and analogs, applied in the field of 2-substituted oxy-5-methylsulfonyl aryl piperazine amides and their preparation, can solve negative symptoms and cognitive symptoms that are not improved, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0106] Example 1: (S)-5-(4-(5-(methylsulfonyl)-2-((1,1,1-trifluoropropan-2-yl)oxo)nicotyryl)piperazine-1 - base)-2,3-dihydro-1H-inden-1-one preparation
[0107] The first step: preparation of (S)-5-(methylsulfonyl)-2-((1,1,1-trifluoropropan-2-yl)oxo)nicotinic acid
[0108]
[0109] Sodium hydride (500 mg, 12.5 mmol) was dissolved in 5 mL of DMF, (S)-trifluoroisopropanol (1.2 g) was added, stirred at room temperature for 30 minutes, and then 2-chloro-5-bromonicotinic acid (900 mg) was added. The system was added, and after stirring at 110 °C for 16 hours, the DMF was spun off, the residue was dissolved in 10 ml of DMSO, and sodium proline (274 mg, 2.0 mmoL), sodium methanesulfinate (1.0 g, 10 mmoL) and iodide were added. Cuprous (761.8 mg, 4.0 mmol), heated to 110 °C and stirred for 4 hours under nitrogen atmosphere, directly separated and purified by reverse phase column chromatography to obtain 5-(methylsulfonyl)-2-((1,1,1- Trifluoropropan-2-yl)oxo)nicotinic acid (150 mg...
Embodiment 2
[0119] Example 2: (S)-(4-(5-Fluoro-3-methylbenzo[d]isoxazol-6-yl)piperazin-1-yl)(5-(methylsulfonyl)-2 Preparation of -((1,1,1-trifluoropropan-2-yl)oxo)phenyl)methanone
[0120] The first step: the preparation of 4-(4-acetyl-2,5-difluorophenyl) piperazine-1-carboxylate tert-butyl ester
[0121]
[0122] Dissolve 2,4,5-trifluoroacetophenone (522 mg, 3.0 mmol) in 10 mL of acetonitrile, then add N-tert-butoxycarbonylpiperazine (671 mg, 3.6 mmol) and potassium carbonate (829 mg, 6.0 mmol), The reaction was heated to reflux overnight. Reversed-phase column chromatography gave the compound 4-(4-acetyl-2,5-difluorophenyl)piperazine-1-carboxylic acid tert-butyl ester (670 mg, 66%). LC-MS:t R =3.12min, [M+H] + 341.2.
[0123] The second step: preparation of tert-butyl 4-(2,5-difluoro-4-(1-(oximinyl)ethyl)phenyl)piperazine-1-carboxylate
[0124]
[0125] 4-(4-Acetyl-2,5-difluorophenyl)piperazine-1-carboxylate tert-butyl ester (670 mg, 1.97 mmol), hydroxylamine hydrochloride (...
Embodiment 3
[0137] Example 3: (S)-(4-(7-Fluoroquinolin-6-yl)piperazin-1-yl)(5-(methylsulfonyl)-2-((1,1,1-trifluoro Preparation of propan-2-yl)oxo)pyridin-3-yl)methanone
[0138] The first step: the preparation of 7-fluoro-6-(piperazin-1-yl)quinoline
[0139]
[0140] Weigh 6-bromo-7-fluoroquinoline (226mg, 1.00mmol), piperazine (129mg, 1.50mmol) and dissolve in 5mL 1,4-dioxane, add bis(tri-tert-butylphosphine) under nitrogen protection Palladium (51 mg, 0.10 mmol) and sodium tert-butoxide (192 mg, 2.00 mmol). Microwave at 120°C for 1 hour. Ethyl acetate was added for extraction, washed with saturated aqueous NaCl solution, and anhydrous Na 2 SO 4 dry. Filtration and concentration gave a tan viscous liquid (210 mg, 91%), and the crude product was directly used in the next reaction.
[0141] The second step: (S)-(4-(7-Fluoroquinolin-6-yl)piperazin-1-yl)(5-(methylsulfonyl)-2-((1,1,1-trifluoro) Preparation of propan-2-yl)oxo)pyridin-3-yl)methanone
[0142]
[0143] Weigh the abo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com