Compound with heterocyclic ligand and preparation method and application thereof
A compound and heterocycle technology, applied in the field of compounds containing heterocycle ligands and its preparation, can solve problems such as poor luminous efficiency and thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0080] The present invention also provides a method for preparing the above compound containing heterocyclic ligands, comprising:
[0081] A) Mixing and reacting the compound having the structure of formula (II) and the compound having the structure of formula (III) to obtain the compound of the structure of formula (IV);
[0082]
[0083] B) reacting the compound with the structure of formula (IV), the compound with the structure of formula (V) and the compound with the structure of formula (VI) to obtain the compound with the structure of formula (I);
[0084] HO——R 10 Formula (Ⅵ);
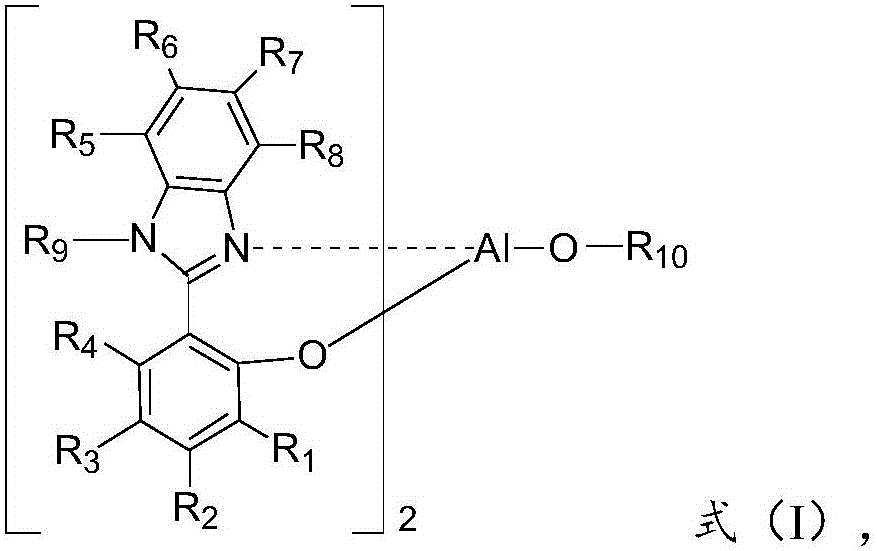
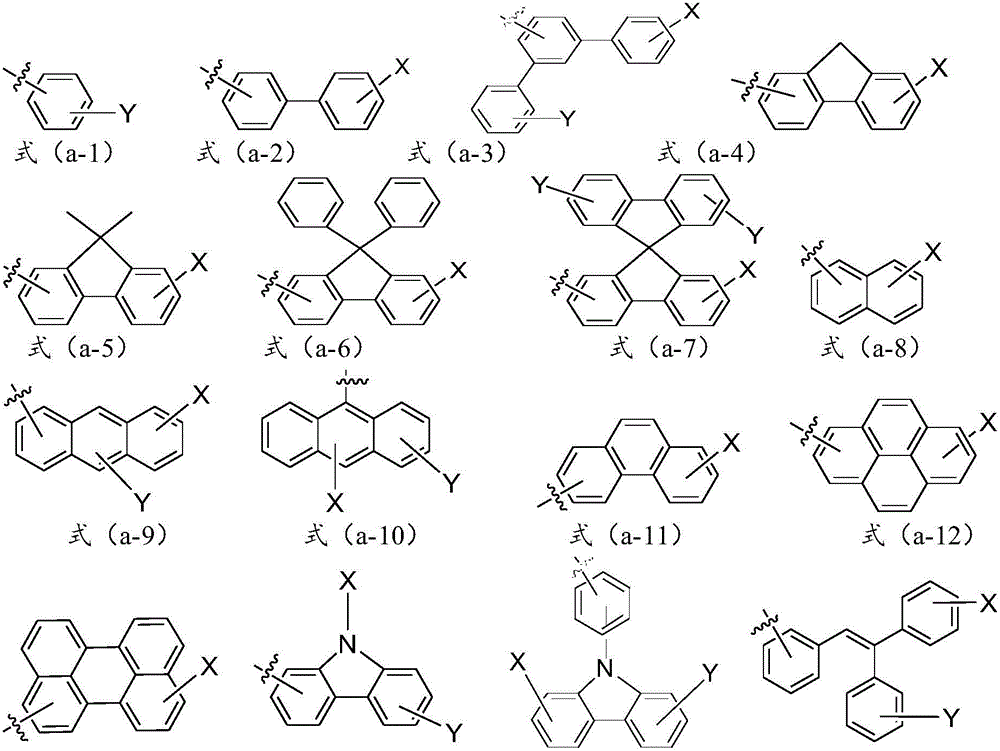
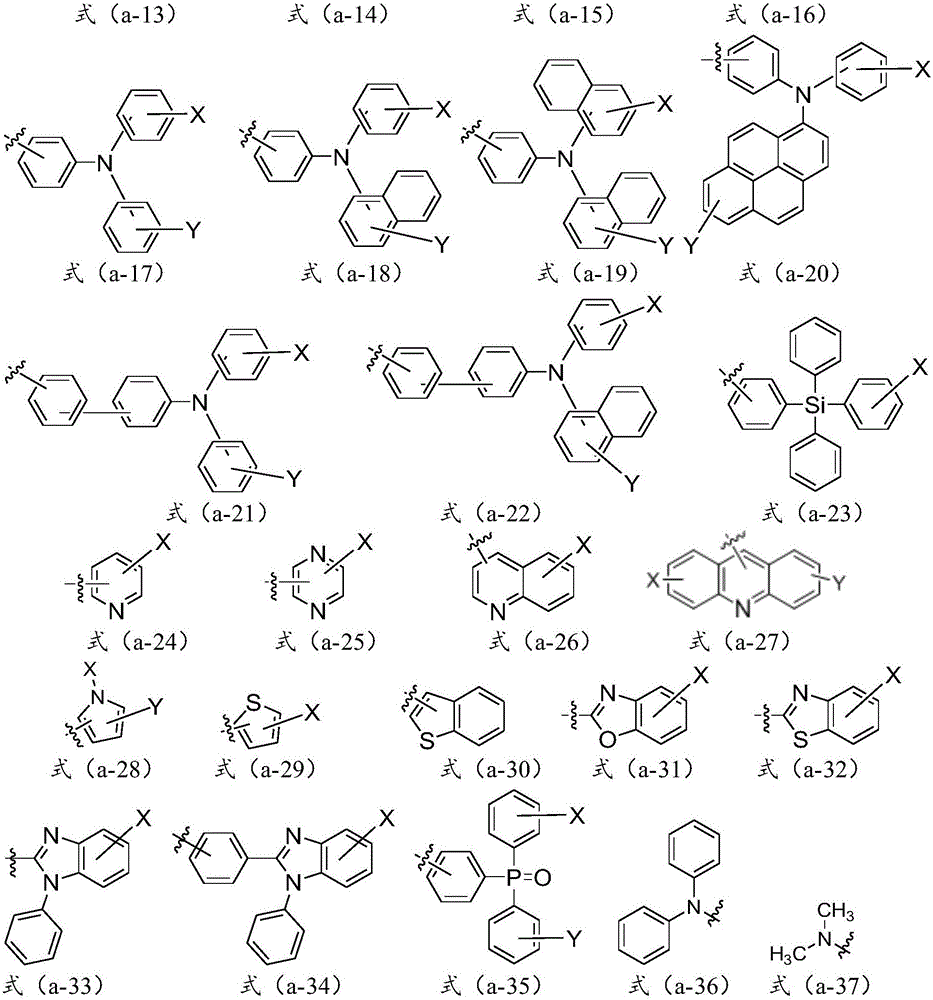
[0085] where the R 1 , R 2 , R 3 , R 4 , R 5 , R 6 , R 7 and R 8 independently selected from hydrogen, halogen, cyano, substituted C1-C30 alkyl, unsubstituted C1-C30 alkyl, substituted C1-C30 cycloalkyl, unsubstituted C1-C30 cycloalkyl , substituted C1-C30 alkoxy, unsubstituted C1-C30 alkoxy, substituted C5-C60 heterocyclic group, unsubstituted C5-C60 heterocyclic group, substitut...
Embodiment 1
[0106] Preparation of Embodiment 1 Intermediate 5-fluoro-2-nitro-N-phenylaniline (formula (II-1-1))
[0107] Add 15.6 g of 5-fluoro-2-aminonitrobenzene into the reaction flask, add 300 mL of anhydrous toluene, stir, add 19.2 g of sodium tert-butoxide, and react for 1 hour under nitrogen protection. Then 30 grams of iodobenzene and 2.8 grams of tris(dibenzylideneacetone) dipalladium were added in sequence, and 4 grams of ligand tri-tert-butylphosphine were added by injection, and the temperature of the reaction was raised to 60° C. overnight.
[0108] After the reaction was completed, the temperature was lowered, 100 mL of water was added to stop the reaction, the water layer was separated, and the toluene layer was dried with anhydrous magnesium sulfate for 30 minutes. The toluene was spin-dried to obtain a dark oil, which was passed through the column, washed with dichloromethane:petroleum ether=1:4, monitored by spot plate, and the product point of RF=0.3 was collected. Aft...
Embodiment 2
[0164] Embodiment 2 has the preparation of the compound of formula (I-2-8) structure
[0165]Add 5 g of the compound having the structure of formula (IV-1-30) into 100 mL of toluene and stir, then add a mixture of 1.8 g of aluminum isopropoxide and water, and stir at 60° C. for 2 h. At the same time, dissolve 1.48g of 4-hydroxybiphenyl in 50mL of methanol and add it dropwise after 2 hours. A white solid precipitates out. After reacting overnight, the white solid is obtained by suction filtration. Rinse with water, then with methanol, and finally with PE to obtain 5g The final product has the compound of formula (I-2-8), yield = 83%. The mass spectral value of this product is 866.29. Above-mentioned reaction process is shown in the following formula:
[0166]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com