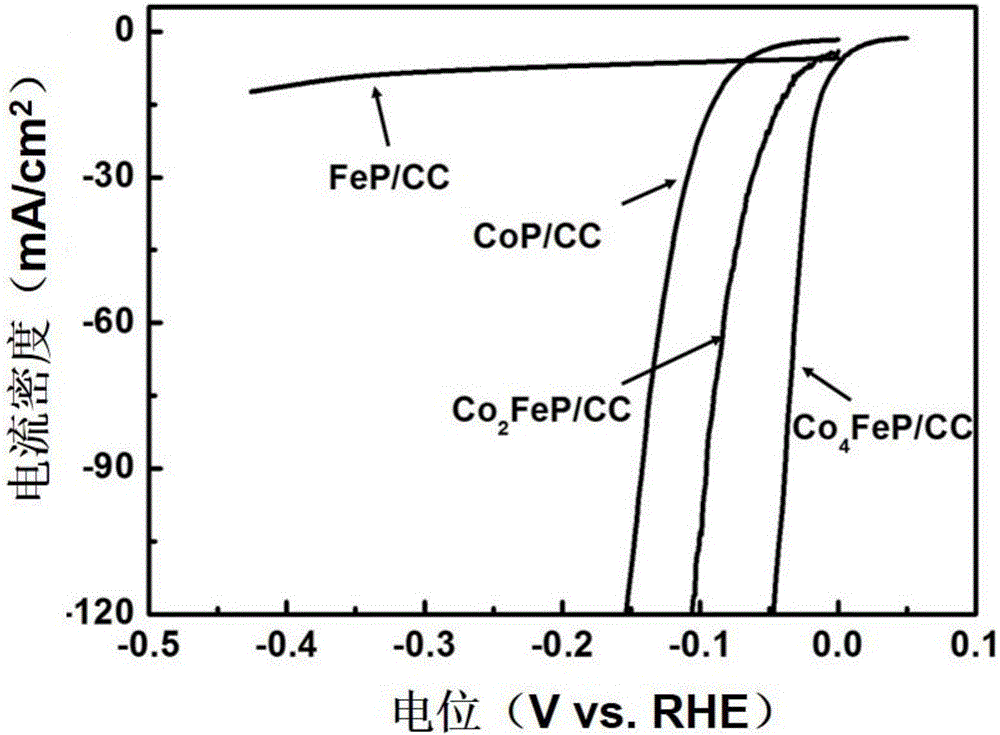
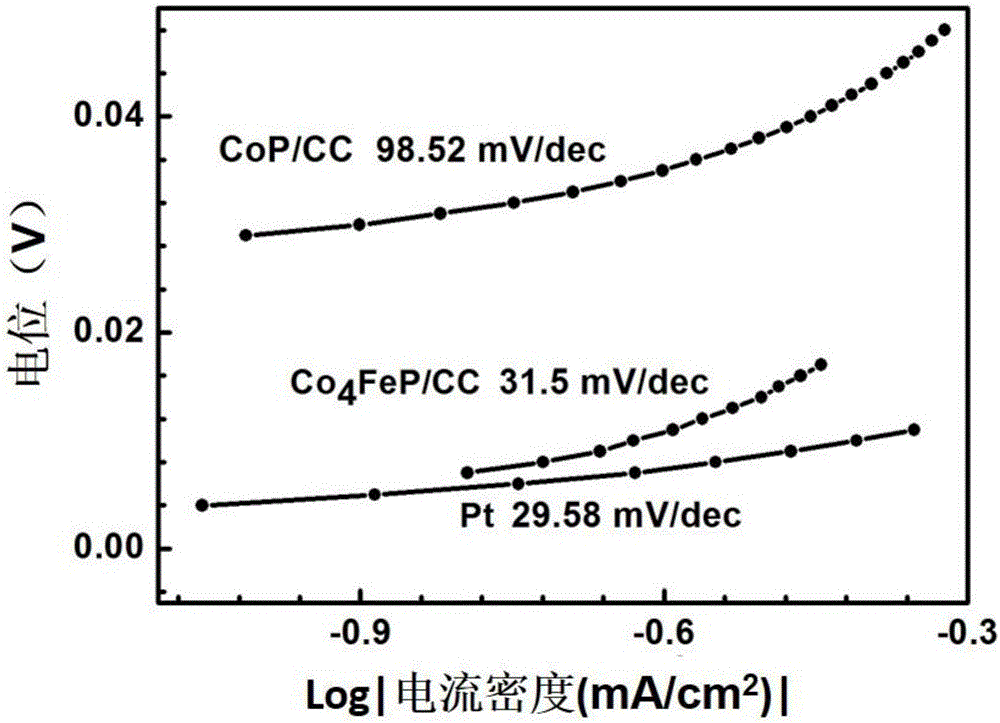
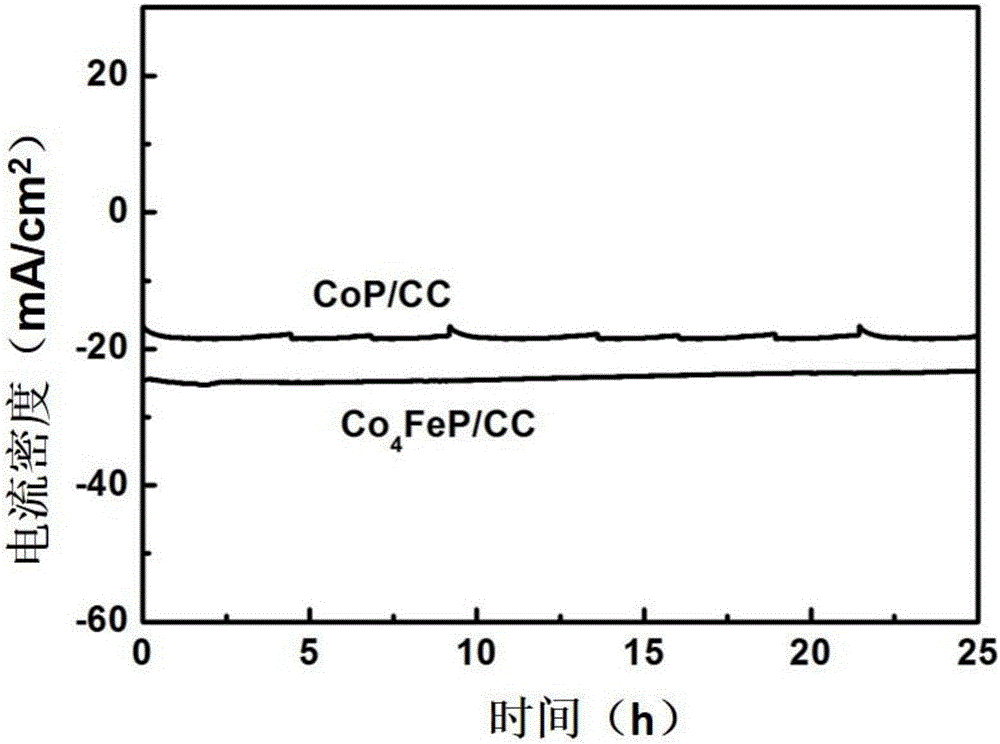
High-efficiency multi-element transition metal phosphide hydrogen-evolution catalyst and preparation method thereof
A transition group metal and catalyst technology, applied in the field of multi-element transition group metal phosphide hydrogen evolution catalyst and preparation, can solve the problems of low hydrogen production, high overpotential and high energy consumption, and achieve reduced hydrogen production cost, good stability, The effect of high catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1, comprises the following steps:
[0022] 1) Prepare the precursor by hydrothermal, add 10mmol cobalt nitrate hexahydrate into 50mL deionized water, stir to form a uniform mixed solution; transfer the mixed solution to a 100mL reactor, add carbon cloth (3×2cm 2 ), completely immerse the carbon cloth in the mixed solution; evenly add 10mL concentrated ammonia water; seal the reaction kettle, heat to 110°C and keep the constant temperature for 9h, take out the carbon cloth and dry it to obtain the precursor;
[0023] 2) Low-temperature phosphorylation of the precursor: NaH 2 PO 2 and the precursor are respectively placed in the upstream and downstream of the tube furnace, and the tube furnace is filled with argon as a protective gas, heated to 300° C. and kept at a constant temperature for 2 hours to obtain a hydrogen evolution catalyst (CoP / CC); the NaH 2 PO 2 The molar ratio with cobalt salt is 3:1.
[0024] Take the catalyst so that its area immersed in...
Embodiment 2
[0025] Embodiment 2, comprises the steps:
[0026] 1) Hydrothermal preparation of the precursor, 8 mmol of cobalt nitrate hexahydrate and 2 mmol of ferric nitrate nonahydrate were added to 50 mL of deionized water, stirred to form a uniform mixed solution; the mixed solution was transferred to a 100 mL reaction kettle, and carbon cloth (3× 2cm 2 ), completely immerse the carbon cloth in the mixed solution; evenly add 10mL of concentrated ammonia water; seal the reaction kettle, heat to 120°C and keep the constant temperature for 10h, take out the carbon cloth and dry it to obtain the precursor;
[0027] 2) Low-temperature phosphorylation of the precursor: NaH 2 PO 2 and the precursor were placed on the upstream and downstream of the tube furnace respectively, and the tube furnace was charged with argon as a protective gas, heated to 300°C and kept at a constant temperature for 2h to obtain a hydrogen evolution catalyst (Co 4 FeP / CC); The NaH 2 PO 2 The molar ratio of coba...
Embodiment 3
[0031] Embodiment 3, comprises the steps:
[0032]1) Prepare the precursor by hydrothermal, add 6.67mmol cobalt nitrate hexahydrate and 3.33mmol ferric nitrate nonahydrate into 50mL deionized water, stir to form a uniform mixed solution; transfer the mixed solution to a 100mL reaction kettle, add carbon cloth ( 3×2cm 2 ), completely immerse the carbon cloth in the mixture; evenly add 10mL of concentrated ammonia water; seal the reaction kettle, heat to 110°C and keep the constant temperature for 9h, take out the carbon cloth and dry it to obtain the precursor;
[0033] 2) Low-temperature phosphorylation of the precursor: NaH 2 PO 2 and the precursor are placed on the upstream and downstream of the tube furnace respectively, the tube furnace is filled with nitrogen or inert gas, heated to 350°C and kept at a constant temperature for more than 2h to obtain a hydrogen evolution catalyst (Co 2 FeP / CC); The NaH 2 PO 2 The molar ratio of cobalt salt and iron salt mixture is 3:1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com