A kind of selective hydrogenation method of C4 fraction
A technology for selective hydrogenation and C4 fractionation, which is applied in the fields of hydrocarbons, chemical instruments and methods, hydrogenation hydrocarbon production, etc., can solve the problems of small liquid space velocity and high cycle ratio of product circulation, so as to improve acid properties and increase Thermal stability and service life extension effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
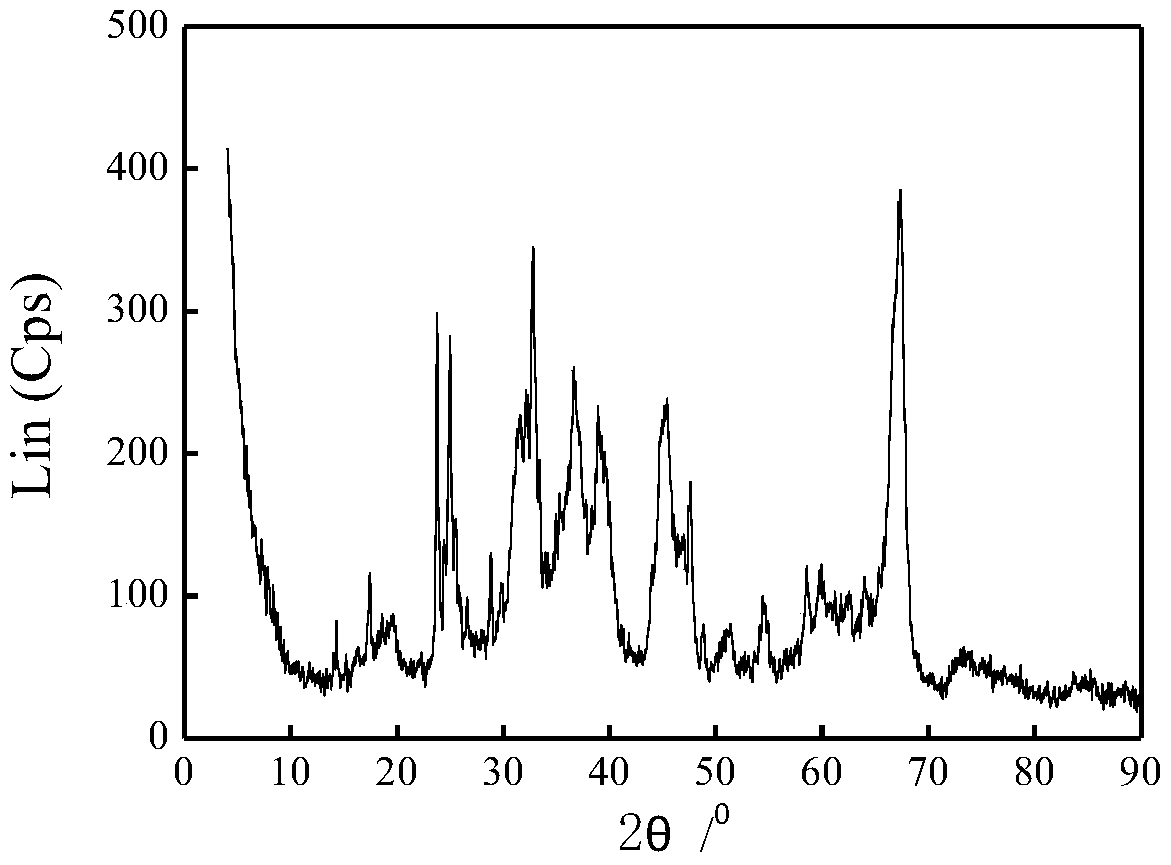
Image
Examples
Embodiment 1
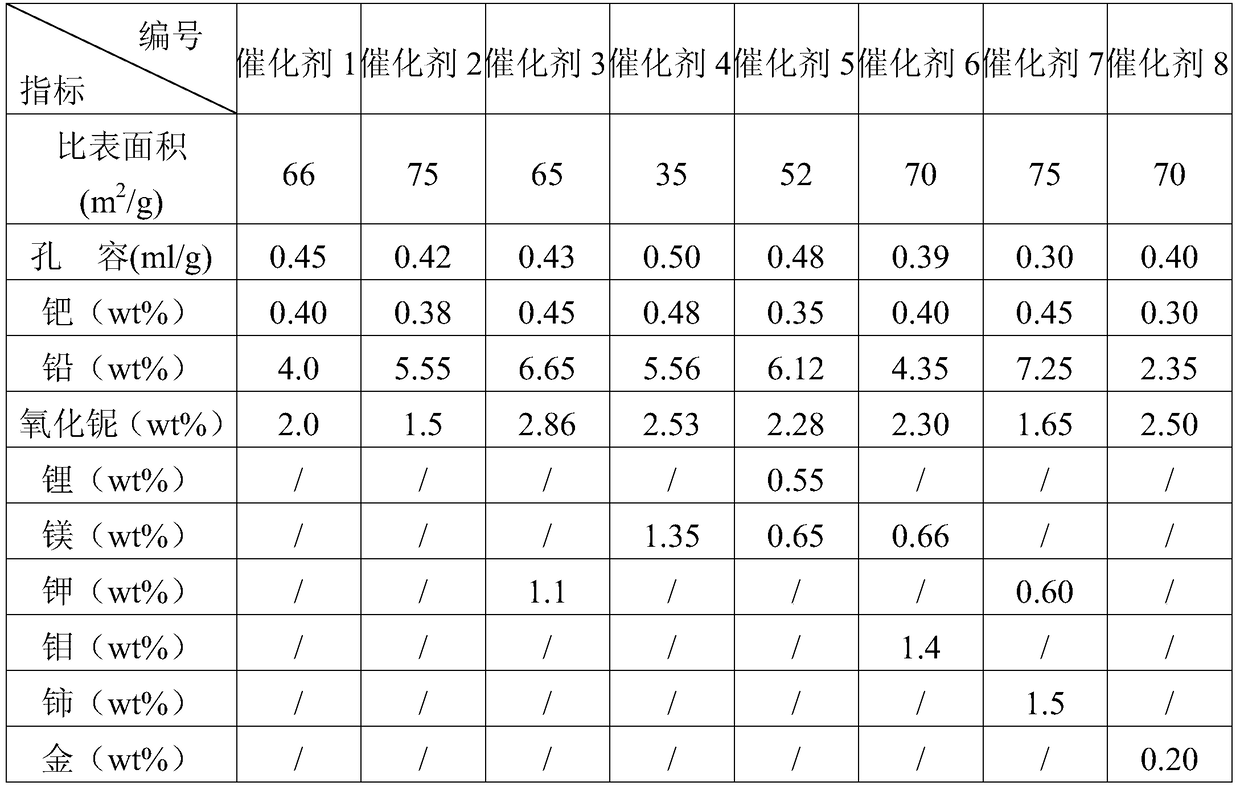
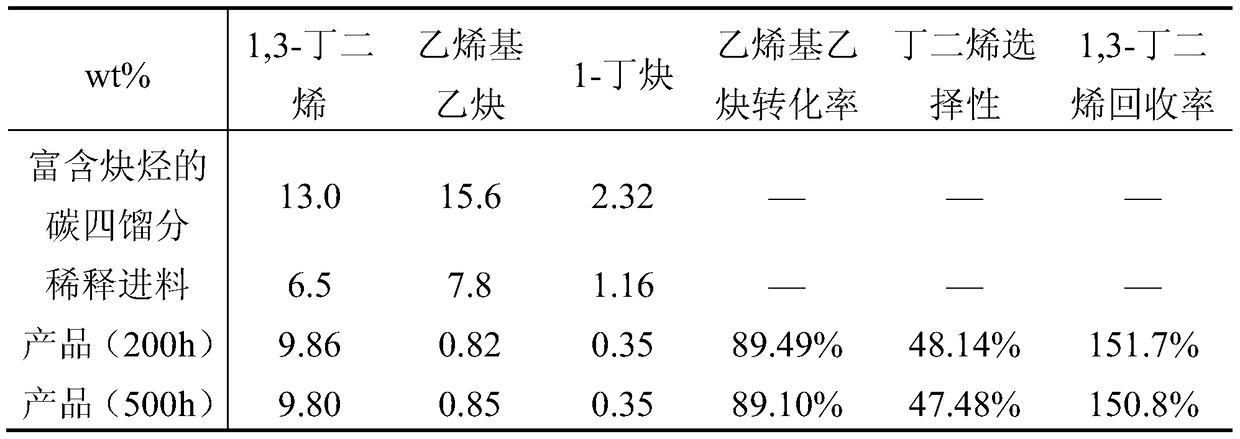
[0065] The C4 fraction rich in alkynes is diluted with the C4 raffinate, and the weight ratio of the C4 fraction rich in alkynes to the C4 raffinate is 1:1. The first stage uses an isothermal bubbling bed reactor and catalyst 1, the reaction temperature is 50°C, and the molar ratio of hydrogen to alkyne is 1.5; the second stage uses an adiabatic bubbling bed reactor and catalyst 2, and the reaction inlet temperature is 40°C , the molar ratio of hydrogen to alkyne is 1.0; the reaction pressure of the first stage and the second stage are both 1.5MPa, and the liquid space velocity is 8h -1 ; Catalysts 1 and 2 were reduced at 120°C for 6h under a hydrogen atmosphere. Table 2 is the composition of materials before and after the reaction.
[0066] Material composition before and after table 2 reaction
[0067]
Embodiment 2
[0069] The C4 fraction rich in alkynes is diluted with the C4 raffinate, and the weight ratio of the C4 fraction rich in alkynes to the C4 raffinate is 1:3. The first stage uses an isothermal bubbling bed reactor with a reaction temperature of 40°C and a molar ratio of hydrogen to alkyne of 1.0; the second stage uses an adiabatic bubbling bed reactor with a reaction inlet temperature of 30°C and The molar ratio is 1.0; the reaction pressure of the first stage and the second stage are both 1.5MPa, and the liquid space velocity is 10h -1 ; Catalyst 3 was used in both reactors, and catalyst 3 was reduced at 120°C for 6h under a hydrogen atmosphere. Table 3 is the composition of materials before and after the reaction.
[0070] Table 3 Composition of materials before and after reaction
[0071]
Embodiment 3
[0073] Dilute the alkyne-rich C4 fraction with raffinate C4, and the weight ratio of the alkyne-rich C4 fraction to the raffinate C4 is 1:2. The first stage uses an isothermal bubbling bed reactor and catalyst 4, the reaction temperature is 50°C, and the molar ratio of hydrogen to alkyne is 2.0; the second stage uses an adiabatic bubbling bed reactor and catalyst 5, and the reaction inlet temperature is 40°C , the molar ratio of hydrogen to alkyne is 1.5; the reaction pressure of the first stage and the second stage are both 2.0MPa, and the liquid space velocity is 12h -1 ; Catalyst 4 and Catalyst 5 were reduced at 120°C for 6h under a hydrogen atmosphere. Table 4 is the composition of materials before and after the reaction.
[0074] Material composition before and after table 4 reaction
[0075]
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com