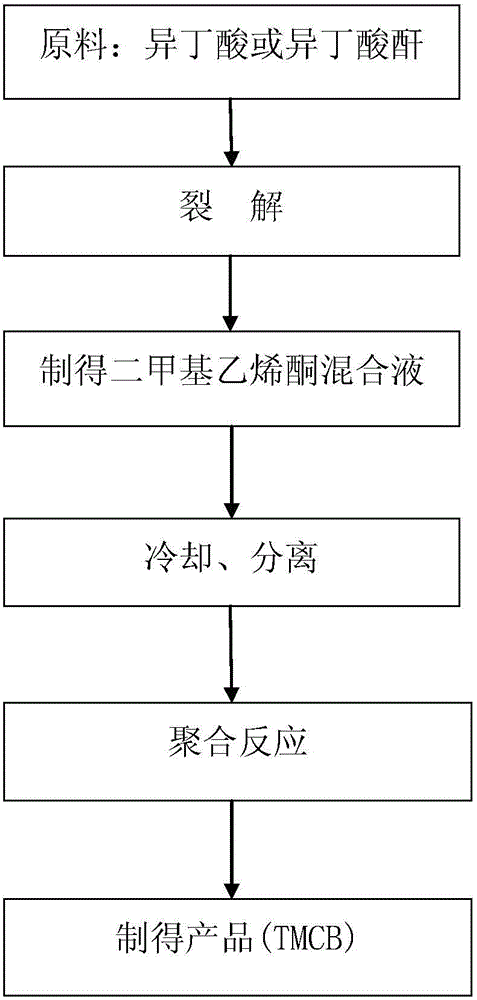
Method for synthesis of 2, 2, 4, 4-tetramethyl-1, 3-cyclobutanedione
A technology of cyclobutanedione and tetramethyl, applied in the field of organic compound preparation, can solve the problems of high cost, difficult to improve the conversion rate of cracking reaction, and many equipments, and achieves the effect of reducing equipment investment, avoiding large consumption and energy loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] 100g of isobutyric acid is fed through a feed pump at a feed rate of 4ml / min, and nitrogen is fed at a feed rate of 20ml / min. After the two are mixed in a mixer, they pass through the cracking reaction device. The cracking temperature is 440°C. The pressure of the reaction process is 3KPa. After the reaction, the reaction product passes through the condenser quickly, and the cooling temperature is controlled at 10°C. The cooled gas-liquid mixture is separated through the separation tank. A 1:1 mixture of dimethyl ester and dimethyl 1,4-cyclohexanedicarboxylate was polymerized at a temperature of 90°C for 60 minutes. 2,2,4,4-Tetramethyl-1,3-cyclobutanedione at a content of 5% (WT) relative to the solvent was obtained by gas chromatography analysis of the absorption liquid.
[0042] The reaction results are as follows:
[0043] Feed rateml / min
Embodiment 2
[0045] 120g of isobutyric anhydride is fed through a feed pump at a feed rate of 6ml / min, nitrogen is fed at a feed rate of 48ml / min, and the two are mixed in a mixer and then passed through the cracking reaction device. The cracking temperature is 480°C. The pressure of the reaction process is 30KPa. The reacted reaction product passes through the condenser quickly, and the cooling temperature is controlled at 20°C. The cooled gas-liquid mixture is separated through the separation tank, and the gas phase product enters the polymerization kettle for reaction first. A 1:2 mixture of dimethyl adipate and dimethyl 1,4-cyclohexanedicarboxylate has been added to the polymerization kettle, and the temperature is controlled at 120°C for 80 minutes of polymerization. By gas chromatography analysis of the absorption solution, 2,2,4,4-tetramethyl-1,3-cyclobutanedione with a content of 15% (WT) relative to the solvent was obtained.
[0046] The reaction results are as follows:
[0047] ...
Embodiment 3
[0049] 200g of isobutyric anhydride is fed through a feed pump at a feed rate of 8ml / min, and nitrogen is fed at a feed rate of 80ml / min. After the two are mixed in a mixer, they pass through the cracking reaction device. The cracking temperature is 520°C. The pressure of the reaction process is 50KPa, the reaction product after the reaction quickly passes through the condenser, the cooling temperature is controlled at 15°C, the cooled gas-liquid mixture is separated through the separation tank, and dimethyl adipate and 1,4 A 1:3 mixed solution of dimethyl cyclohexanedicarboxylate was polymerized at a controlled temperature of 120°C for 80 minutes. By gas chromatography analysis of the absorption solution, 2,2,4,4-tetramethyl-1,3-cyclobutanedione with a content of 15% (WT) relative to the solvent was obtained.
[0050] The reaction results are as follows:
[0051] Feed rateml / min
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com