Synthetic method of alkyl acid testosterone compound
A synthesis method and compound technology, applied in the synthesis field of alkyl acid testosterone compounds, can solve the problems of environmental pollution, toxicity, increase cost and the like, and achieve the effect of improving preparation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
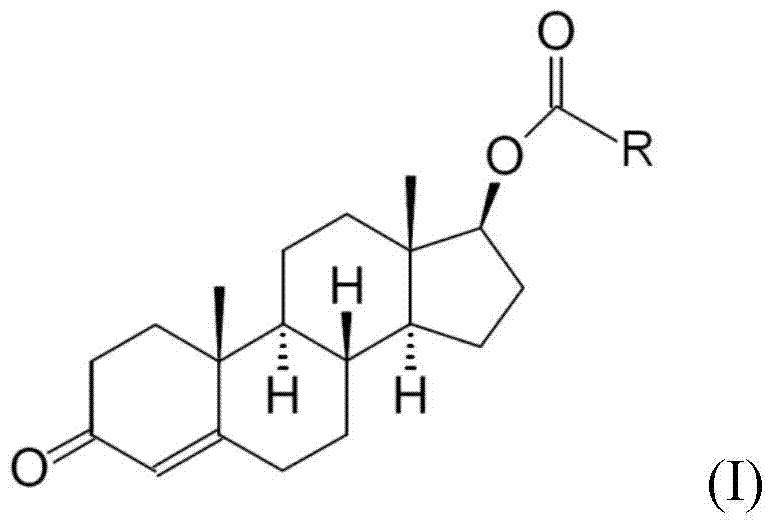
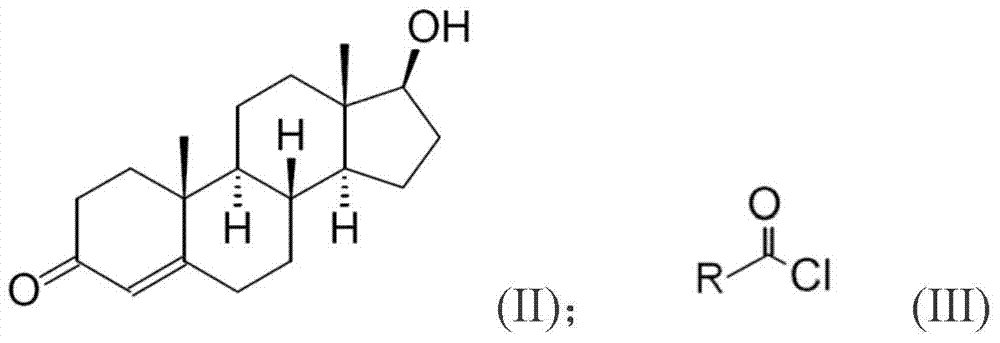
[0025] Embodiment 1-7 is to provide the detailed synthesis method of testosterone undecanoate (Testosterone undecanoate), is to use testosterone (Testosterone) as starting material to carry out esterification reaction, and this esterification reaction is as shown in the following formula program:
[0026]
[0027] First, under nitrogen, 5g of testosterone was thoroughly mixed with 25ml of N,N-dimethylformamide (DMF, solvent) and 5ml of pyridine (pyridine, base), and then 4.6ml of Undecanoylchloride (Undecanoylchloride), and react at room temperature for 1 hour, after the reaction, add 5ml of water and stir for 1 hour, the product precipitates and is filtered, and then rinses the product with acetone aqueous solution and then vacuum-dries it at room temperature , to obtain 7.6 g of testosterone undecylate, the yield was 96%, HPLC: 98.26%.
[0028] Next, the recrystallization step is to add 120g of testosterone undecylate into 480ml of acetone, stir until it is completely dis...
Embodiment 2
[0030] This example is roughly the same as Example 1, the difference lies in the types of solvents used and the steps of subsequent recrystallization are different. In this example, 25ml of N,N-dimethylacetamide was used as a solvent, and 7.6g of testosterone undecylate was obtained after the reaction, with a yield of 96%, HPLC: 99.40%.
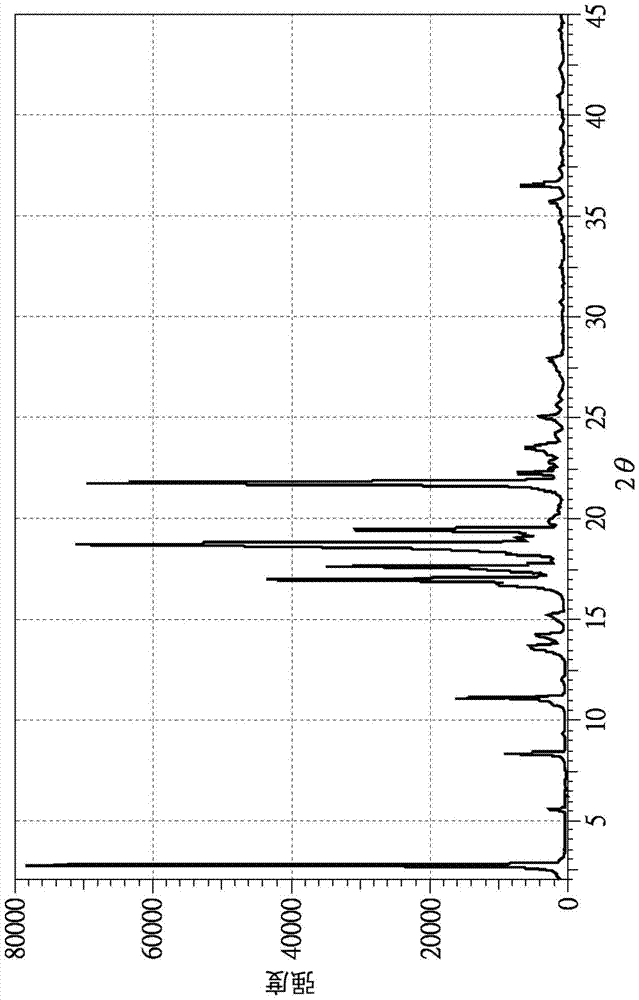
[0031] Next, the recrystallization step of this embodiment is to add 6.1g of testosterone undecylate into 61ml of ethanol, stir until completely dissolved, stir in an ice bath for 1 hour, filter and rinse with ethanol, and The testosterone undecanoate obtained after recrystallization is Form S crystal.
Embodiment 3
[0033] First, 5 g of testosterone was thoroughly mixed with 50 ml of acetonitrile (solvent) and 1.7 ml of pyridine (pyridine, base) under nitrogen, and then 4.6 ml of undecanoyl chloride was added at 0-10° C. Under reaction for 5 hours, after the reaction ended, add 10ml of water and stir for 0.5 hours to separate out the product and filter, then rinse the product with acetone aqueous solution and vacuum-dry it at room temperature to obtain 5.5g of testosterone undecylate , the yield was 69%, HPLC: 98.80%.
[0034] Next, the recrystallization step is to add 3g of testosterone undecylate into 15ml of DMF, stir at 35°C until completely dissolved, then add 1ml of water and stir for 0.5 hours, filter and rinse with DMF aqueous solution Yes, the testosterone undecylate obtained after recrystallization is the S crystal form (Form S).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com