A miRNA-182 inhibitor and its application in the preparation of drugs for preventing and treating immune rejection in heart transplantation
A technology of mirna-182 and 1. mirna-182 is applied in the field of medical bioengineering to achieve the effect of protecting the function of the heart graft and preventing the failure of the heart graft
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
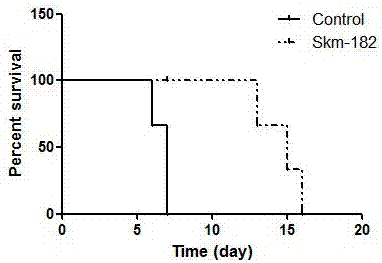
[0020] Example 1: Detecting the Effect of miRNA-182 Inhibitors on the Survival Time of Transplanted Hearts Using a Mouse Model of Heterotopic Heart Transplantation
[0021] 1. Synthesis of the miRNA-182 inhibitor of the present invention
[0022] 5'-O-(4,4-dimethoxytrityl)-2'-O-methylcytidine-3'-(2-cyanoethyl-N,N-diisopropyl )(5'-O-(4,4'-dimethoxytrityl)-2'-O-methyl-3'-O-(2-cyanoethyl-N,N-diisopropyl)) modified RNA phosphoramidite monomer A, G, C, U, using a DNA synthesizer to chemically synthesize the miRNA-182 inhibitor of the present invention (abbreviated as Skm-182 in the schematic diagram), the RNA sequence of the miRNA-182 inhibitor is 5'-CGGUGUGAGUUCUACCAUUGCCAAA-3'. The connection modification of RNA and cholesterol groups was carried out in a controlled-pore glass solid support. During the synthesis of miRNA-182 inhibitors, the modification of the phosphorosulfuryl bond at the designated position is accomplished by the oxidation reaction of phosphite and phenylacet...
Embodiment 2
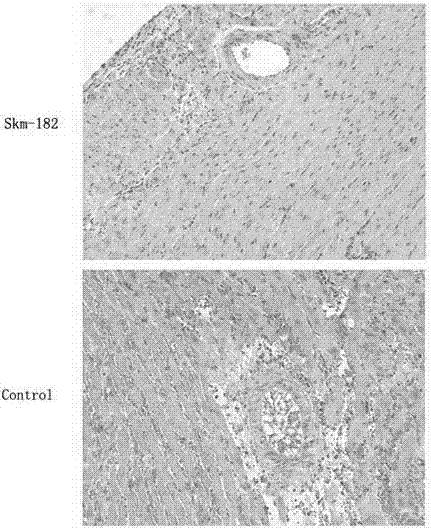
[0032] Embodiment 2: Observing the pathological changes of the heart graft with the method of HE staining
[0033] 1. Experimental method: 1) on the 7th day after transplantation, each 3 recipient mice in the control group and the experimental group were sacrificed to obtain transplanted heart tissue pieces, which were fixed with 10% neutral formalin for 12- After 24 hours, they were routinely embedded in paraffin and sectioned at 4 μm with a Leica microtome. 2) Sections were dewaxed with xylene and washed with various levels of ethanol to water. The dewaxing steps are: xylene (I) 5 min→xylene (II) 5 min→100% ethanol for 2 min→95% ethanol for 1 min→80% ethanol for 1 min→75% ethanol for 1 min→distilled water washing for 2 min. 3) Harris hematoxylin semen was stained for 5 minutes, and washed with tap water for 1 minute. 4) Differentiate in hydrochloric acid ethanol solution for 30s. 5) Soak in tap water for 15 minutes or warm water (about 50°C) for 5 minutes. 6) Place the e...
Embodiment 3
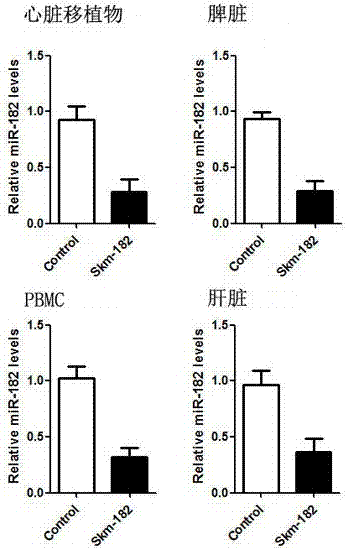
[0035] Example 3: Detection of expression changes of miRNA-182 in mouse in vivo tissues by fluorescent quantitative PCR
[0036] 1. Extraction of Total miRNAs from Heart Grafts, Spleen, Liver, and PBMCs
[0037] On the 7th day after transplantation, 3 recipient mice in each of the control group and the experimental group were sacrificed, and part of the heart graft, spleen and liver tissues were removed respectively. At the same time, mouse whole blood was collected in EP tubes containing EDTA anticoagulant for the extraction of peripheral blood mononuclear cells (PBMC). Total miRNAs were then extracted from heart grafts, spleen, liver and PBMCs, respectively.
[0038] (1) Extraction of PBMC and its miRNA
[0039] PBMCs were separated by Ficoll density gradient centrifugation. First add 2ml of lymphocyte separation medium to a 15ml centrifuge tube, mix 1ml of blood and 1ml of 1640 medium evenly, slowly add to the upper layer of lymphocyte separation medium, centrifuge at 20...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com