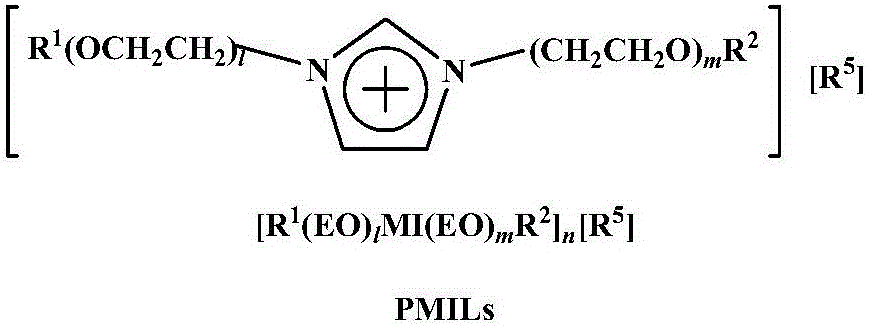
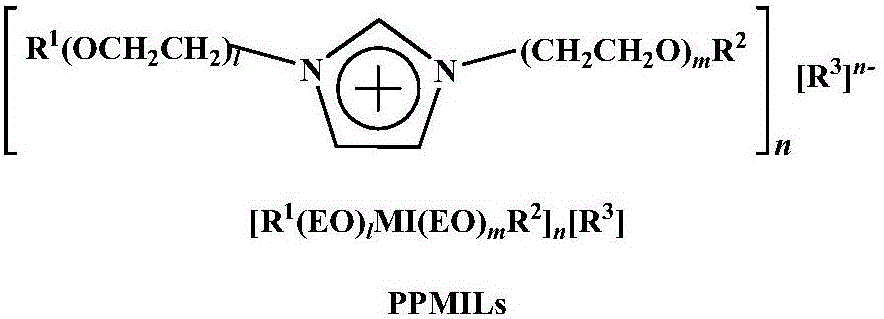Method for high-selectivity preparation of normal aldehyde through olefin two-phase hydroformylation on basis of phosphine-functionalized polyether imidazolium salt ionic liquid
A polyether imidazolium salt and ionic liquid technology, applied in the direction of carbon monoxide reaction preparation, chemical instruments and methods, organic compound/hydride/coordination complex catalyst, etc., can solve the problem of low catalytic activity and poor regioselectivity of normal aldehydes , High dosage of ionic liquid, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
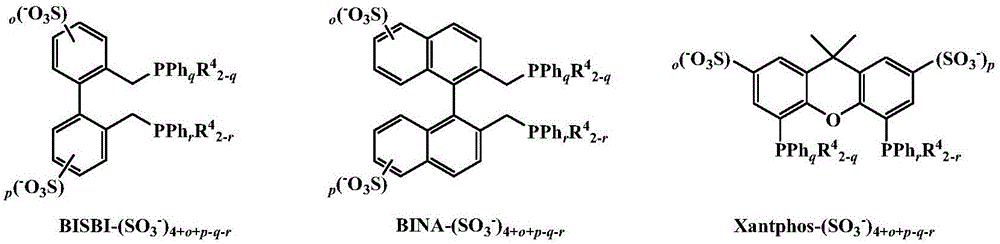
[0026] Rh(acac)(CO) 2 / BISBI-(SO 3 Na) 2 (o=p=1, q=r=2) / [Ph(EO) 16 MI(CH 3 )][CH 3 SO 3 - ] / 1-octene two-phase hydroformylation reaction
[0027] Under an inert atmosphere, add Rh(acac)(CO) to a stainless steel autoclave 2 , BISBI-(SO 3 Na) 2 , [Ph(EO) 16 MI(CH 3 )][CH 3 SO 3 - ] and 1-octene, the ratio is: BISBI-(SO 3 Na) 2 / Rh(acac)(CO) 2 =5:1 (molar ratio), 1-octene / Rh(acac)(CO) 2 =1000:1 (molar ratio), [Ph(EO) 16 MI(CH 3 )][CH 3 SO 3 - ] / Rh(acac)(CO) 2 =300:1 (molar ratio), then use synthesis gas (H 2 / CO=1:1) pressurized to 5.0MPa, reaction temperature 100°C, reaction time 0.5 hours, then rapidly cooled to room temperature, vented the synthesis gas and opened the kettle, realized rhodium catalyst by two-phase separation of ionic liquid phase and organic phase The recovery of n-heptane can also be added for extraction, and the organic phase containing product aldehyde is obtained through simple two-phase separation. The gas chromatography analysis ...
Embodiment 2
[0029] Rh(acac)(CO) 2 / BINA-(SO 3 Na) 2 (o=p=1, q=r=2) / [Ph(EO) 16 MI(CH 3 )][CH 3 SO 3 - ] / 1-octene two-phase hydroformylation reaction
[0030] Under an inert atmosphere, add Rh(acac)(CO) to a stainless steel autoclave 2 , BINA-(SO 3 Na) 2 , [Ph(EO) 16 MI(CH 3 )][CH 3 SO 3 - ] and 1-octene in a ratio of: BINA-(SO 3 Na) 2 / Rh(acac)(CO) 2 =5:1 (molar ratio), 1-octene / Rh(acac)(CO) 2 =5000:1 (molar ratio), [Ph(EO) 16 MI(CH 3 )][CH 3 SO 3 - ] / Rh(acac)(CO) 2 =300:1 (molar ratio), then use synthesis gas (H 2 / CO=1:1) pressurized to 5.0MPa, reaction temperature 100°C, reaction time 0.5 hours, then rapidly cooled to room temperature, vented the synthesis gas and opened the kettle, realized rhodium catalyst by two-phase separation of ionic liquid phase and organic phase The recovery of n-heptane can also be added for extraction, and the organic phase containing product aldehyde can be obtained through simple two-phase separation. The gas chromatography analysis...
Embodiment 3
[0032] Rh(acac)(CO) 2 / Xantphos-(SO 3 Na) 2 (o=p=1, q=r=2) / [Ph(EO) 16 MI(CH 3 )][CH 3 SO 3 - ] / 1-octene two-phase hydroformylation reaction
[0033] Under an inert atmosphere, add Rh(acac)(CO) to a stainless steel autoclave 2 , Xantphos-(SO 3 Na) 2 , [Ph(EO) 16 MI(CH 3 )][CH 3 SO 3 - ] and 1-octene in a ratio of: Xantphos-(SO 3 Na) 2 / Rh(acac)(CO) 2 =5:1 (molar ratio), 1-octene / Rh(acac)(CO) 2 =1000:1 (molar ratio), [Ph(EO) 16 MI(CH 3 )][CH 3 SO 3 - ] / Rh(acac)(CO) 2 =300:1 (molar ratio), then use synthesis gas (H 2 / CO=1:1) pressurized to 5.0MPa, reaction temperature 100°C, reaction time 0.5 hours, then rapidly cooled to room temperature, vented the synthesis gas and opened the kettle, realized rhodium catalyst by two-phase separation of ionic liquid phase and organic phase The recovery of n-heptane can also be added for extraction, and the organic phase containing product aldehyde is obtained through simple two-phase separation. The gas chromatography ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com