Ion conductivity test apparatus and test method employing same
A technology of ionic conductivity and testing device, applied in the direction of measurement device, measurement of electrical variables, measurement of resistance/reactance/impedance, etc., can solve the problem of inability to separate electronic conductivity, etc., and achieve the effect of accurate measurement method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
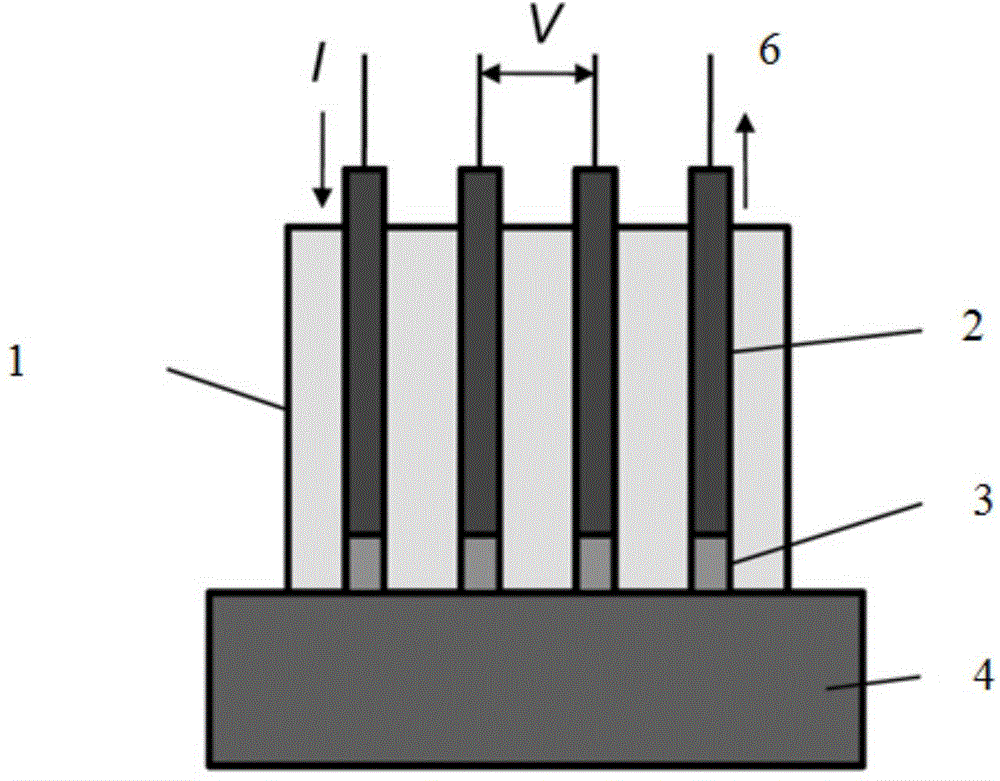
[0028] The schematic diagram of the test device is as figure 1 As shown, on a cylindrical polytetrafluoroethylene block with a diameter of 2 cm, 4 through holes arranged linearly along the central axis are machined, each with a diameter of 1 mm and a distance between the origins of 3 mm. Fix four platinum wires with the same diameter as the circular hole in the hole, and the distance between the end face of the platinum wire and the end face of the polytetrafluoroethylene block is 1 mm. Drop-coat 5% Nafion polyion solution on the end surface of the platinum wire, and apply it repeatedly after drying until the solid Nafion polymer completely covers the end surface of the platinum wire.
[0029] The test circuit is that the middle two platinum wires are externally connected to the voltage test terminals of the potentiostat (reference electrodes 1 and 2), and the two outer platinum wires are externally connected to the current test terminals (working electrode and counter electro...
Embodiment 2
[0038] The testing device is the same as that in Example 1.
[0039] a. Measurement of sample ion conductance:
[0040] Divide the moistened size to 5 x 5cm 2 The Nafion115 membrane and the above-mentioned test electrode have one end of the Nafion polymer tightly pressed against the surface of the sample to be tested. Use a potentiostat to apply a linear potential signal at the voltage test terminal, the voltage range is -1 to 1V, and record the current response signal at the current test terminal.
[0041] b. Data processing:
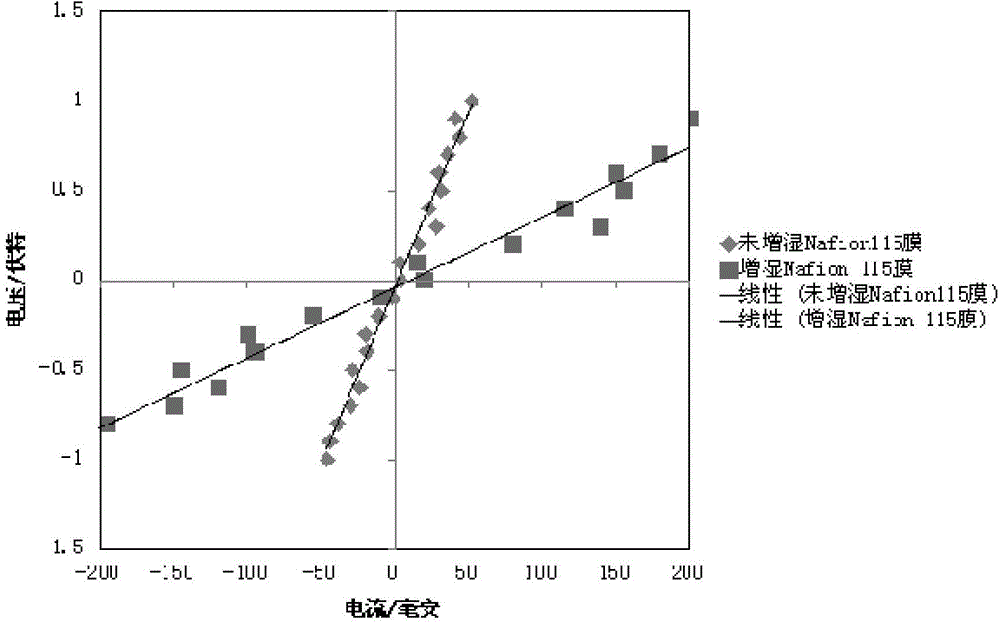
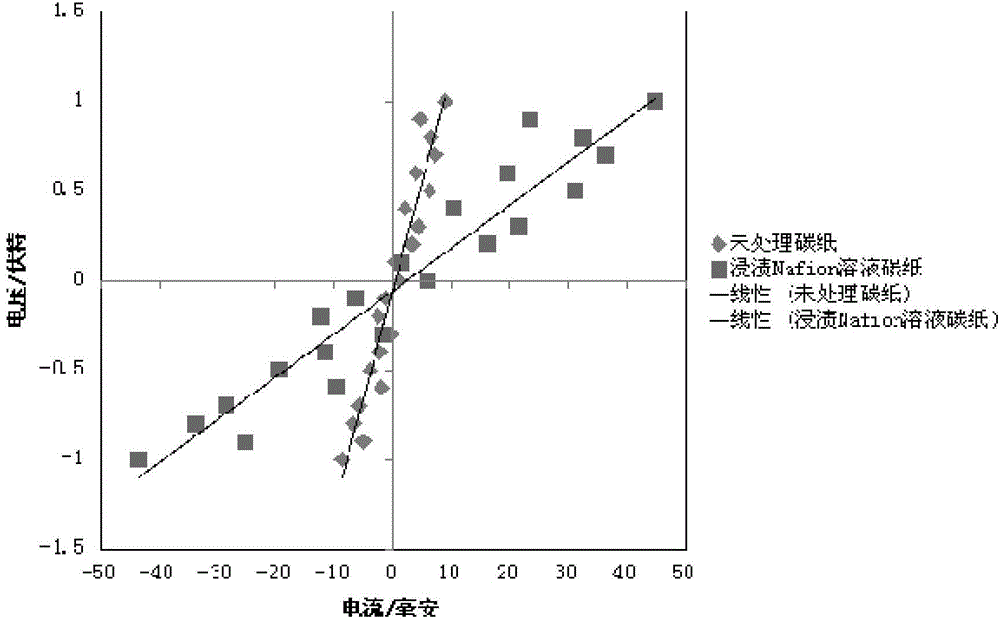
[0042] Taking the above-mentioned measured current signal as the abscissa and the voltage as the ordinate to plot, an approximate linear curve can be obtained, such as figure 2 shown. A linear fitting is carried out at a section with better linearity near the zero potential, and the slope d of the obtained fitting curve is the test ionic resistance of the sample to be tested.
[0043] According to the geometric structure of the probe electrode us...
Embodiment 3
[0047] The testing device is the same as that in Example 1.
[0048] a. Measurement of sample ion conductance:
[0049] Dimensions will be 5×5cm 2 The carbon paper and the above-mentioned test electrode have one end of the Nafion polymer tightly pressed against the surface of the sample to be tested. Use a potentiostat to apply a linear potential signal at the voltage test terminal, the voltage range is -1 to 1V, and record the current response signal at the current test terminal.
[0050] b. Data processing:
[0051] Taking the above-mentioned measured current signal as the abscissa and the voltage as the ordinate to plot, an approximate linear curve can be obtained, such as figure 2 shown. A linear fitting is carried out at a section with better linearity near the zero potential, and the slope d of the obtained fitting curve is the test ionic resistance of the sample to be tested.
[0052] According to the geometric structure of the probe electrode used in the test and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com