Dry powder inhaler of interferon
A dry powder inhaler and interferon technology, which is applied in powder delivery, antiviral agent, aerosol delivery, etc. It can solve the problems of dose discount, inability to accurately control the dose, and low effective inhalation dose.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1: Preparation of Interferon Dry Powder Inhaler Granules
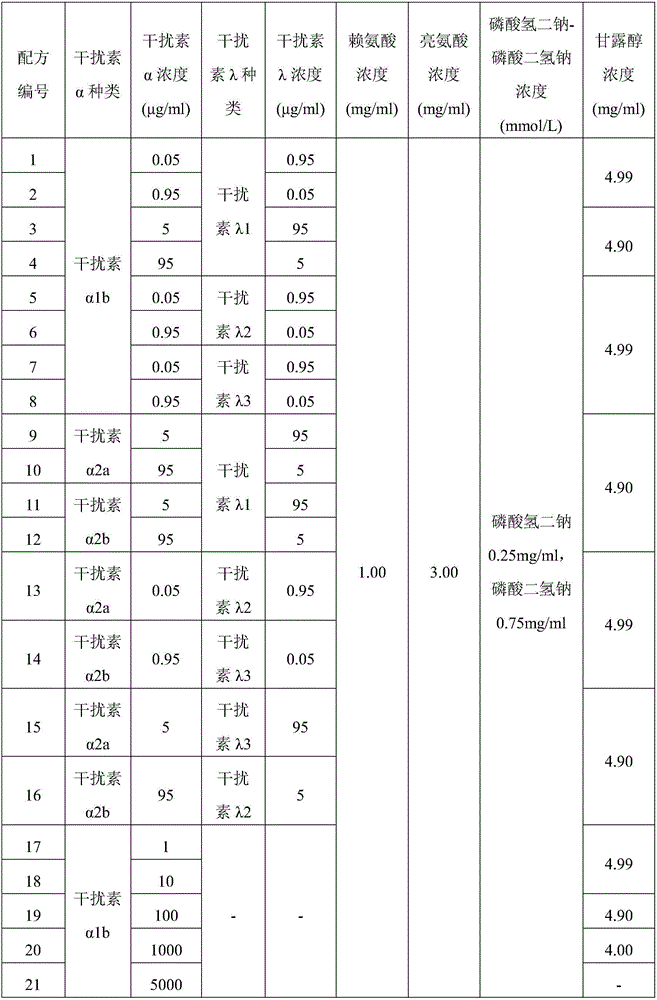
[0051] Each spray-drying solution is prepared according to the formula in the following table 1 (wherein the main function of lysine is the interferon active protective agent, the main function of leucine is dispersibility auxiliary, disodium hydrogen phosphate-sodium dihydrogen phosphate The main function is pH stabilizer (adjusting pH is 7.0), and the main function of mannitol is diluent), and the solution is spray-dried with BuchiB-290 spray dryer according to the corresponding spray drying conditions in Table 2 (after all liquid sprays are completed) Continue to maintain the spray drying gas inlet temperature and flow rate for 15 minutes), thereby preparing the interferon dry powder inhaler particles.
[0052] Table 1 prepares the solution formula for spray drying of interferon dry powder inhaler particles
[0053]
[0054]
[0055] The spray drying conditions of table 2 interferon dry powder in...
Embodiment 2
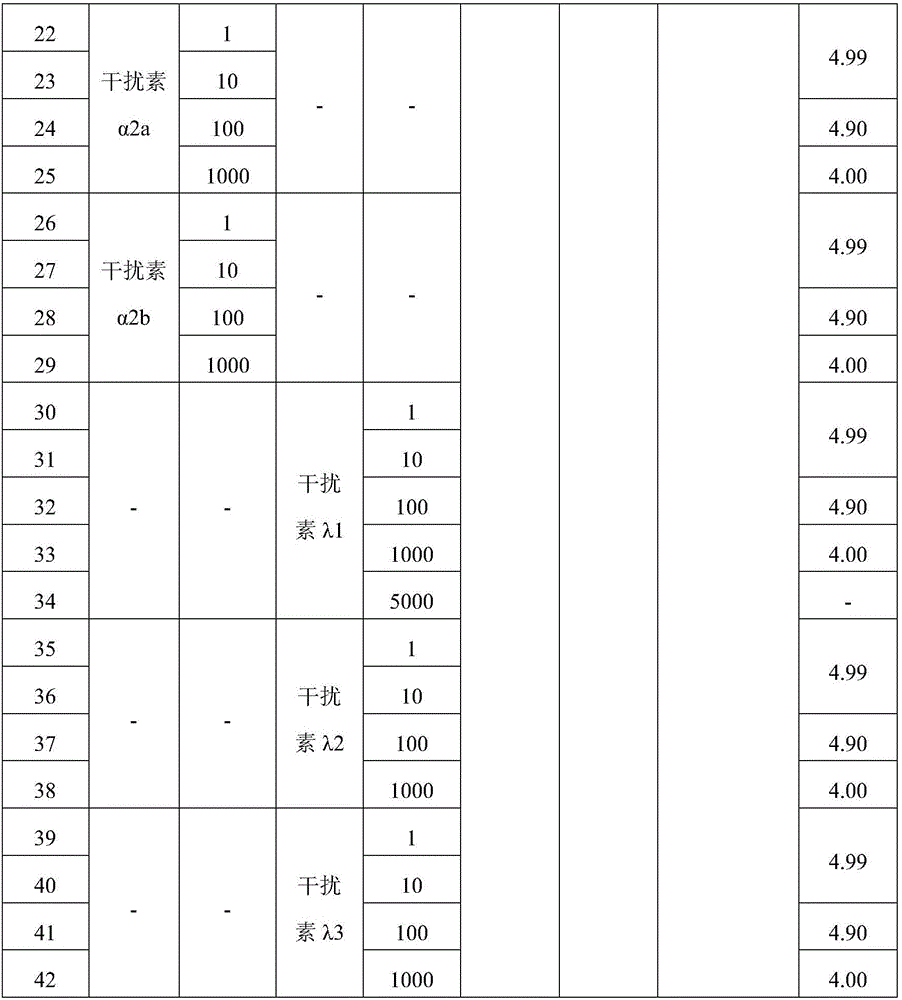
[0057] Example 2: Quality evaluation of interferon dry powder inhaler particles
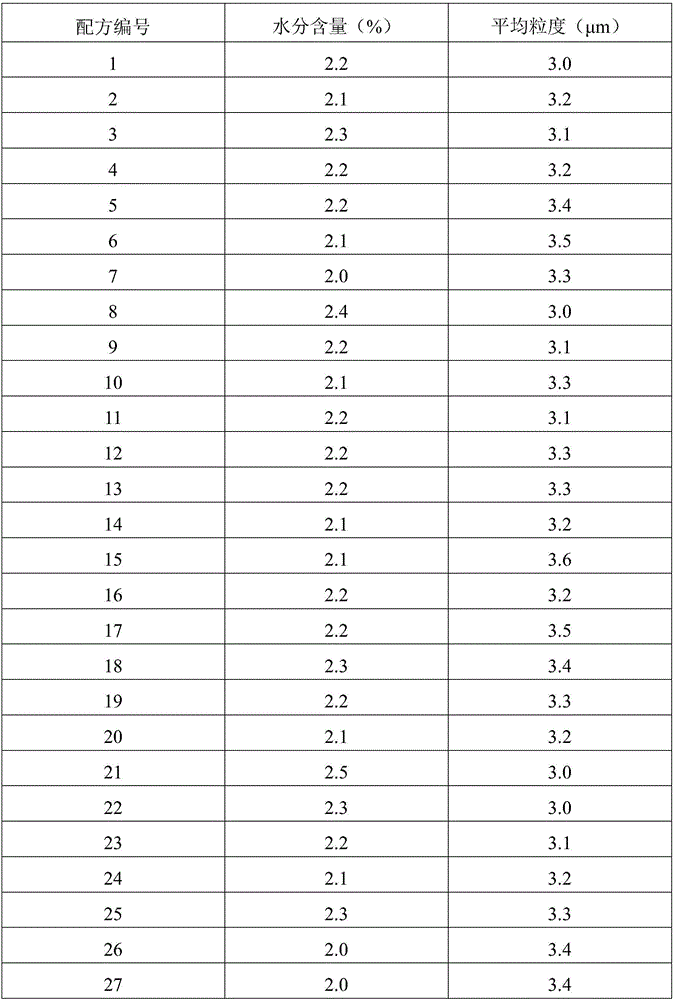
[0058] The moisture content in the prepared interferon dry powder inhaler granules of each formula was determined according to the provisions of the first method of "Moisture Determination Method" in the appendix of "Pharmacopoeia of the People's Republic of China 2010 Edition (Part Three)".
[0059] The average particle size of the prepared interferon dry powder inhaler particles of each formula was determined according to the provisions of the first method of "Particle Size and Particle Size Distribution Determination Method" in the appendix of "Pharmacopoeia of the People's Republic of China 2010 Edition (Part II)".
[0060] All measurement results are shown in Table 3 below.
[0061] The quality evaluation results of the interferon dry powder inhaler particles prepared by table 3 spray drying
[0062]
[0063]
Embodiment 3
[0064] Example 3: Preparation of Interferon Dry Powder Inhaler from Interferon Dry Powder Inhaler Granules
[0065] The interferon dry powder inhaler particles of formula 1-42 obtained by the method of Example 1 are mixed with commercially available large-size lactose carrier particles in a mass ratio of 1:1, respectively, and are packaged in an amount of 20 mg per capsule. The interferon dry powder inhalers of formula 1'-42' were then used for the research of Example 4 respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com