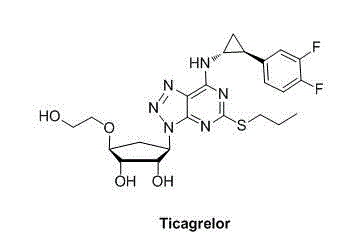
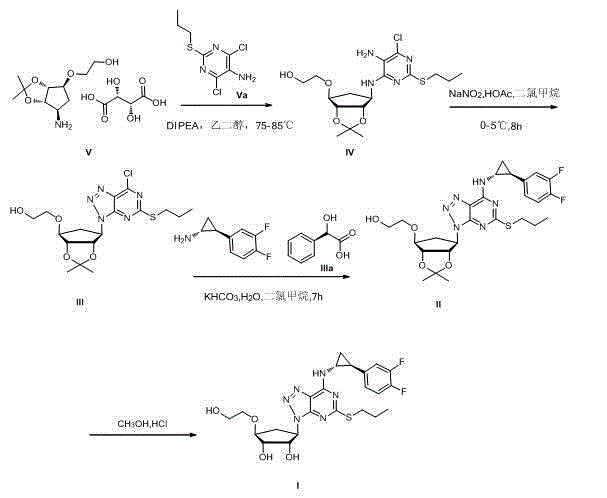
Purification method of ticagrelor
A technology of ticagrelor and purification method, applied in the preparation of ticagrelor, in the field of pharmaceutical industry, can solve problems such as no research and reports, and achieve the effects of easy control operation, high product purity, and low purification cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The homemade 100g crude ticagrelor (HPLC purity 95.5%, isomer 1.3%) and 500g ethyl acetate were heated to 50oC and stirred to dissolve. Heat filtration, collect filtrate, add 1000g normal hexane in filtrate. After the addition, lower the temperature and stir to crystallize until a large amount of white solid is precipitated, and keep stirring at 10oC to 15oC for 1 hour. After filtering, the filter cake was rinsed with a mixed solution of 150 g of ethyl acetate and 300 g of n-hexane. After vacuum drying at 40oC for 8 hours, 87g of off-white solid was obtained. The HPLC purity was 99.89%, isomer 0.003%, the largest single impurity amount was 0.005%, and the total impurity amount was 0.011%.
Embodiment 2
[0026] 100g of self-made crude ticagrelor (HPLC purity 95.5%, isomer 1.3%) and 600g of acetone were heated to 35oC and stirred to dissolve. Heat filtration, collect filtrate, add 1800g isooctane in filtrate. After the addition, cool down and stir to crystallize until a large amount of white solids are precipitated, then keep stirring at 5oC to 10oC for 2 hours. After filtering, the filter cake was rinsed with 200g of acetone and 600g of isooctane mixture. Vacuum drying at 45oC for 10 hours yielded 83 g of off-white solid with HPLC purity of 99.80%, isomer 0.006%, maximum single impurity amount 0.008%, and total impurity amount 0.020%.
Embodiment 3
[0028] 100g of self-made crude ticagrelor (HPLC purity 95.5%, isomer 1.3%) and 700g of n-butanol were heated to 55oC and stirred to dissolve. Heat filtration, collect filtrate, add 2800g n-hexane in filtrate. After the addition, cool down and stir to crystallize until a large amount of white solids are precipitated, and keep stirring at 15oC to 20oC for 2 hours. After filtering, the filter cake was rinsed with a mixture of 230g n-butanol and 920g n-hexane. After vacuum drying at 50oC for 12 hours, 83 g of off-white solid was obtained, with HPLC purity of 99.76%, isomer 0.008%, maximum single impurity amount 0.009%, and total impurity amount 0.024%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com