Triterpene-2-alpha-hydroxylase MAA45, related biological materials thereof and application of triterpene two-bit alpha-hydroxylase MAA45 and related biological materials in preparing maslinic acid and corosolic acid
A technology of α-hydroxylase, biomaterials, applied in the field of biology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
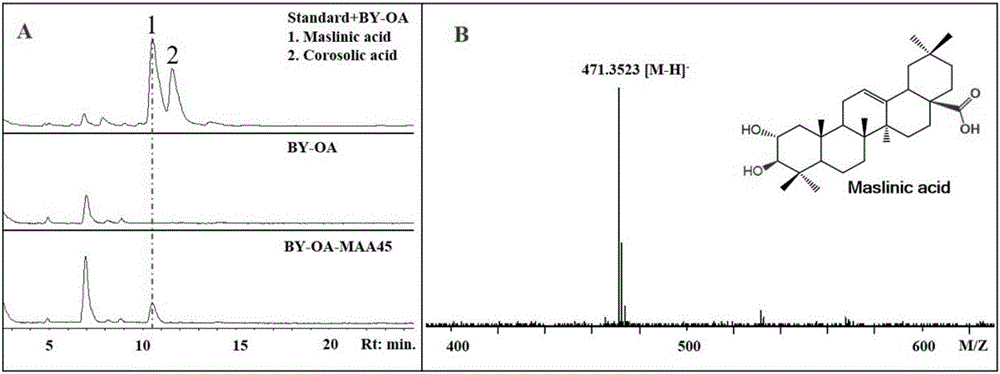
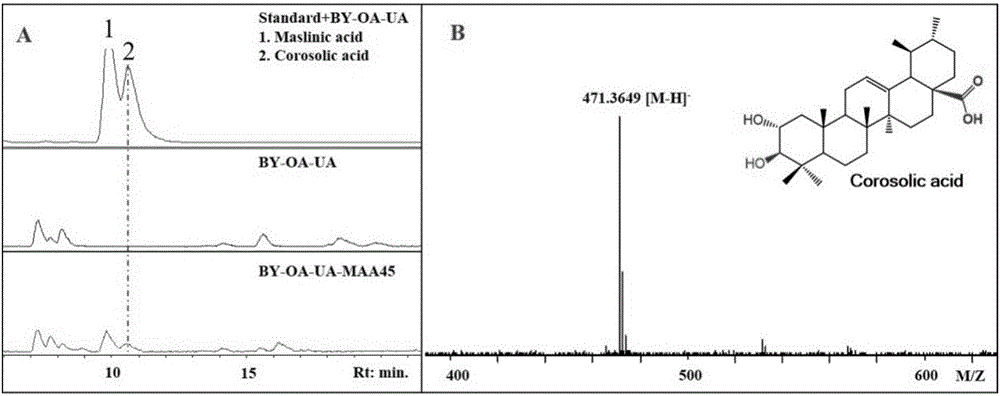
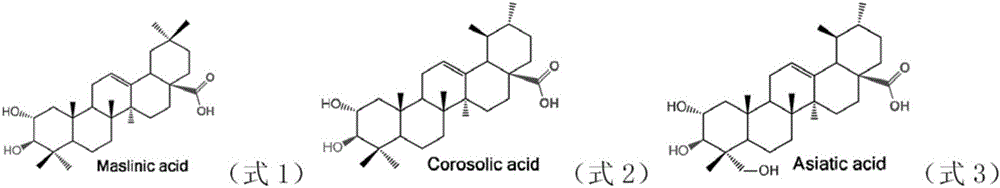
[0082] Example 1, MAA45 can be used to prepare maslinic acid and corosolic acid
[0083] The present invention provides a protein from hawthorn (Crataegus pinnatifida Bunge), whose name is maslinic acid / corosolic acid-related protein (MAA45). MAA45 can catalyze the α-hydroxylation of the 2-position of triterpene, and belongs to the 2-position α-hydroxylation of triterpene. The enzyme, the amino acid sequence of MAA45 is shown in sequence 1 in the sequence listing, which can be encoded by the MAA45 gene shown in the 795-2225th position of sequence 2.
[0084] 1. Preparation of recombinant vector
[0085] Using the restriction endonuclease SacII, insert the DNA fragment shown in the 7-2540th position of sequence 2 between the SacII recognition sequence of the vector pRS313, keep the other sequences of the vector pRS313 unchanged, obtain the recombinant vector, and name the recombinant vector is pRS313-HIS-PGK1-MAA45-CYC1t.
[0086]Among them, the 1-6th and 2541-2546th position...
PUM
| Property | Measurement | Unit |
|---|---|---|
| percent by volume | aaaaa | aaaaa |
| percent by volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com