Metal hydrides coordination catalyst and preparation and application thereof
A coordination catalyst and double metal cyanide technology, which is applied in the preparation of metal cyanide coordination catalysts and the preparation of new metal cyanide coordination catalysts, can solve problems that do not involve the chemical modification of the active center of the catalyst. , to achieve the effect of improving the degradation performance and reducing the degree of reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
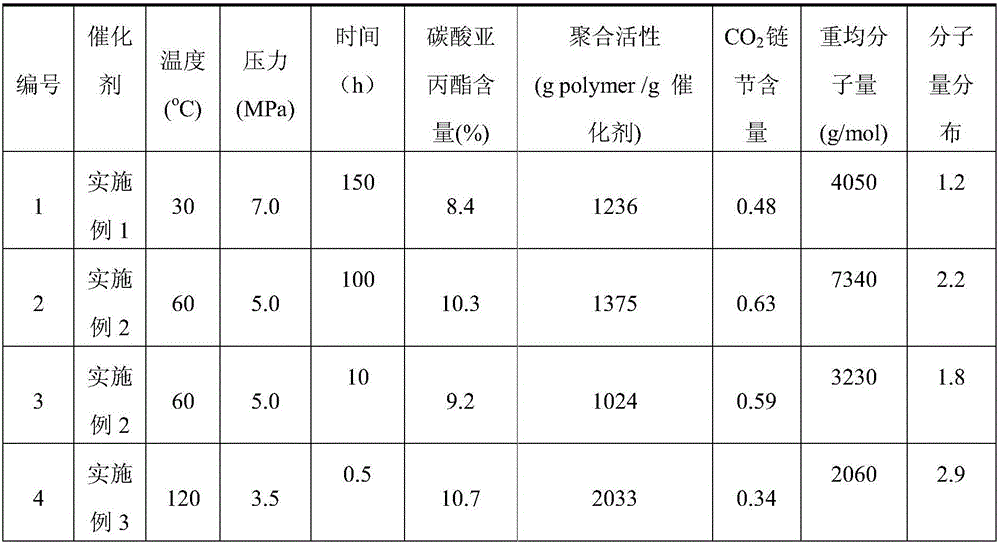
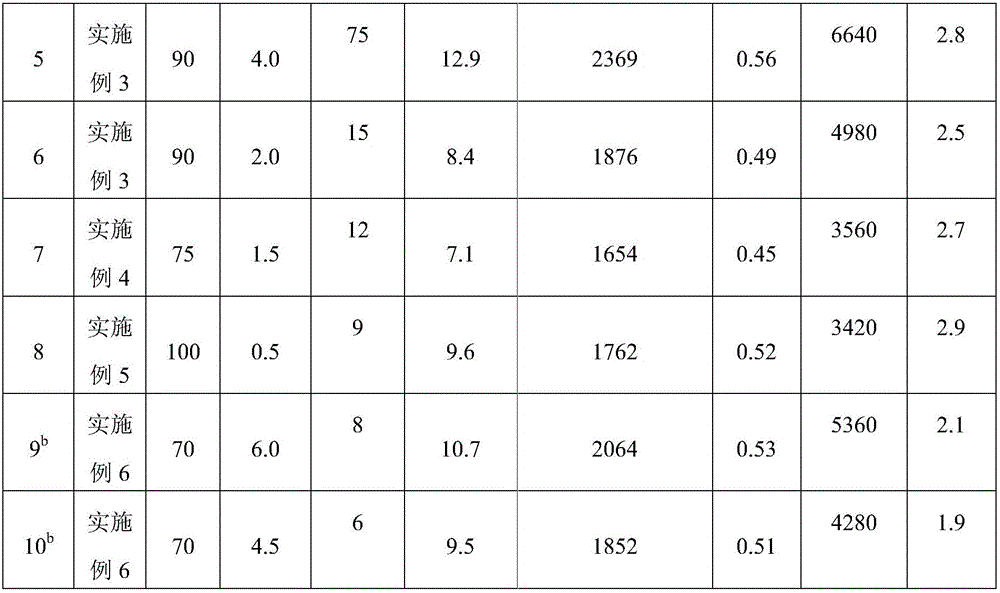
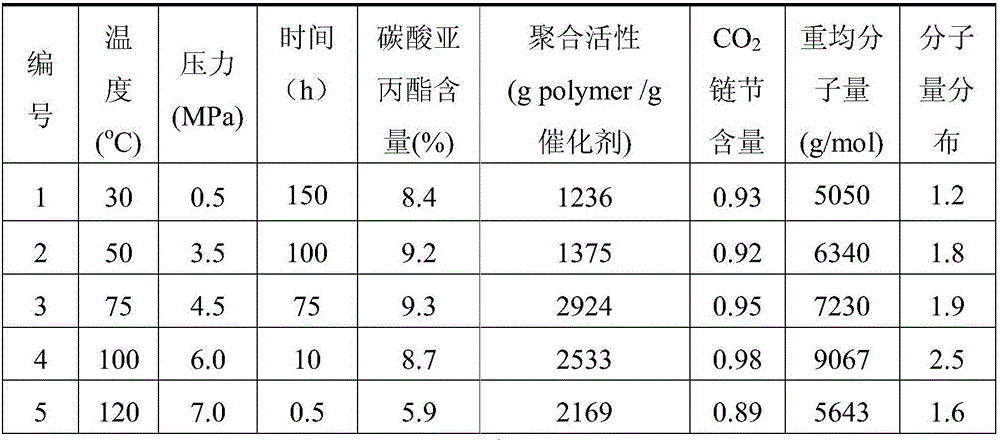
[0044] First, 1.00 g of Zn-Co(III) DMCC described in formula (2) was dried at a temperature of 130° C. under vacuum for 20 hours. TGA results showed that water and tert-butanol in the catalyst could be removed, and the structural formula was Zn 2 [Co(CN) 6 ](OH) 0.6 Cl 0.4 . Then add 309mL acetic acid to the dry complex under a dry argon atmosphere, and react with magnetic stirring at 20°C for 200 hours. Dry in a vacuum oven to constant weight to obtain 0.92 g of a white solid, which is sealed and stored.
Embodiment 2
[0046] First, 0.50 g of Zn-Co(III) DMCC described in formula (2) was dried at a temperature of 190° C. under vacuum for 8 hours. TGA results showed that the water and tert-butanol in the catalyst were completely removed, and the structural formula was Zn 2 [Co(CN) 6 ](OH) 0.6 Cl 0.4 . Then 22.06 g of trichloroacetic acid was added, dissolved in n-hexane and reacted with magnetic stirring at 70° C. for 100 hours. After the reaction, filter with suction, wash the filter cake three times with 300 mL of n-hexane, and dry the solid filter cake in a vacuum oven at 60°C until constant weight to obtain 0.41 g of white solid, which is sealed and stored.
Embodiment 3
[0048] First, 0.20 g of Zn-Co(III) DMCC described in formula (2) was dried at a temperature of 250° C. under vacuum for 2 hours. TGA results showed that the water and tert-butanol in the catalyst were completely removed, and the structural formula was Zn 2 [Co(CN) 6 ](OH) 0.6 Cl 0.4 . Then 7.7g of propiolic acid was added, dissolved in cyclohexane and reacted with magnetic stirring at 120°C for 0.5 hours. After the reaction, filter with suction, wash the filter cake three times with 300 mL of cyclohexane, and dry the solid filter cake in a vacuum oven at 60°C to constant weight to obtain 0.16 g of white solid, which is sealed and stored.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com