Method for synthesizing hydroxyacetate through reactive rectification catalyzed by solid acid
A technology of glycolic acid ester and glycolic acid, which is applied in the field of synthesizing glycolic acid ester by reactive distillation catalyzed by solid acid, can solve the problems of long process flow, complicated purification process and equipment of synthetic raw material gas, limited application and development, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
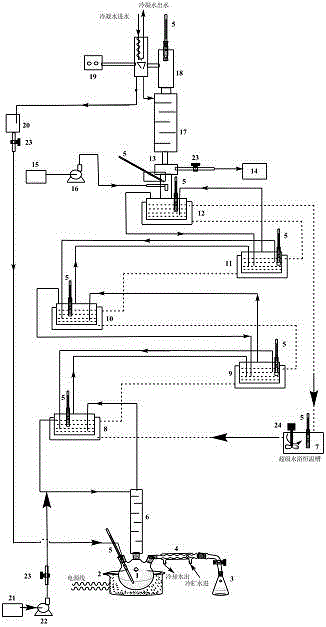
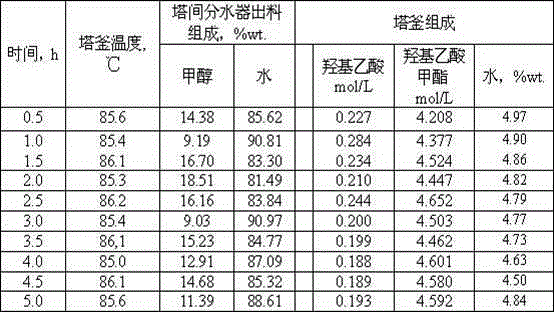
[0023]Add 500mL of pure methanol solution into a 1000mL tower kettle (four-necked flask 1), and add 100mL glycolic acid methanol ethanol eluate into five 150mL jacketed reactors (8, 9, 10, 11, 12), respectively, where The molar ratio of methanol / glycolic acid is 5:1, and then 50g of treated and dried 732-type cation exchange resin (for the treatment method, please refer to the common conventional acid-base treatment method for ion exchange resins) is added to the five reactors as a solid acid catalyst. Open the condensed water valve of the tower top condenser 18, and feed cooling water; turn on the heating power supply of the tower kettle electric heating jacket 2, and carry out the temperature rise of the tower kettle 1 feed liquid; turn on the super water bath constant temperature tank 7 power supply for heating, adjust the temperature of the water bath and keep it constant at Around 80°C. In the experiment, the entire reactive distillation column can be allowed to react in...
Embodiment 2
[0031] The experimental raw materials and experimental conditions were the same as in Example 1, and the catalyst was changed to D001 type cation exchange resin. After the reaction, the total amount of feed liquid was 600 mL, which contained 175.8 g of glycolic acid. The total output of the tower kettle is 514.5g (volume 490mL), which contains 0.3677mol / L of glycolic acid and 4.227mol / L of methyl glycolate. Based on this calculation, the conversion rate of glycolic acid is 92.21%. The yield is 89.56%.
Embodiment 3
[0033] The catalyst used was 732 type cation exchange resin, and the experimental raw material was ethanol alcohol eluting liquid. Other experimental conditions were similar to those in Example 1. After the reaction, the total amount of feed liquid was 600mL, which contained 163.2g of glycolic acid. The total output of the tower kettle is 520.8g (volume 496mL), which contains 0.2913mol / L of glycolic acid and 4.032mol / L of ethyl glycolate. Based on this calculation, the conversion rate of glycolic acid is 93.27%. The yield is 93.15%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com