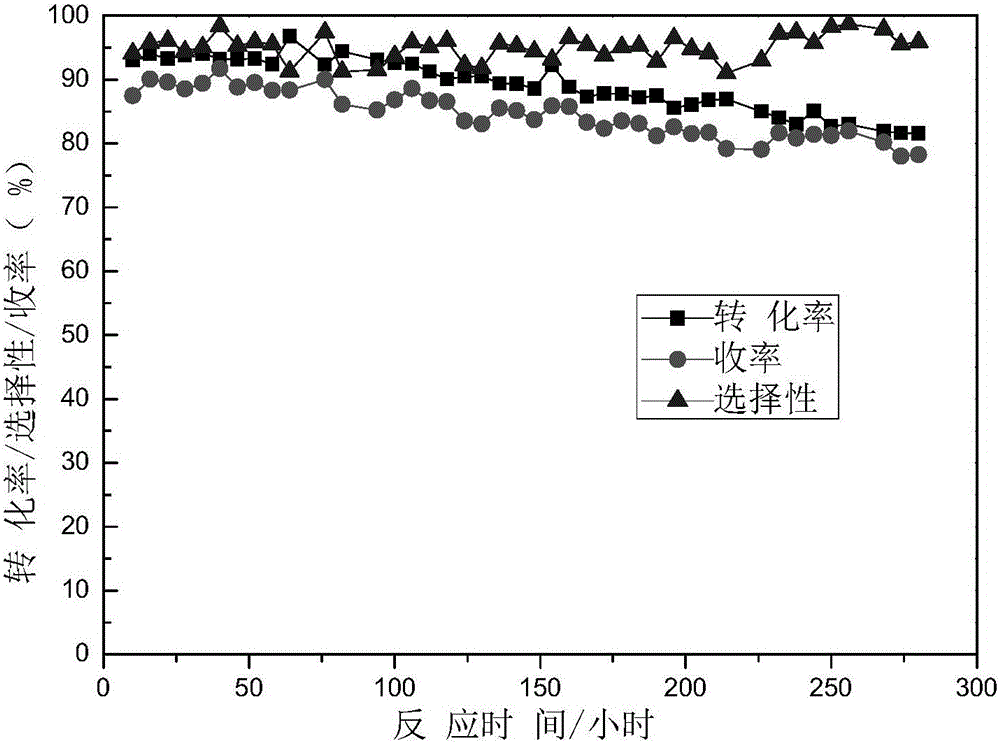
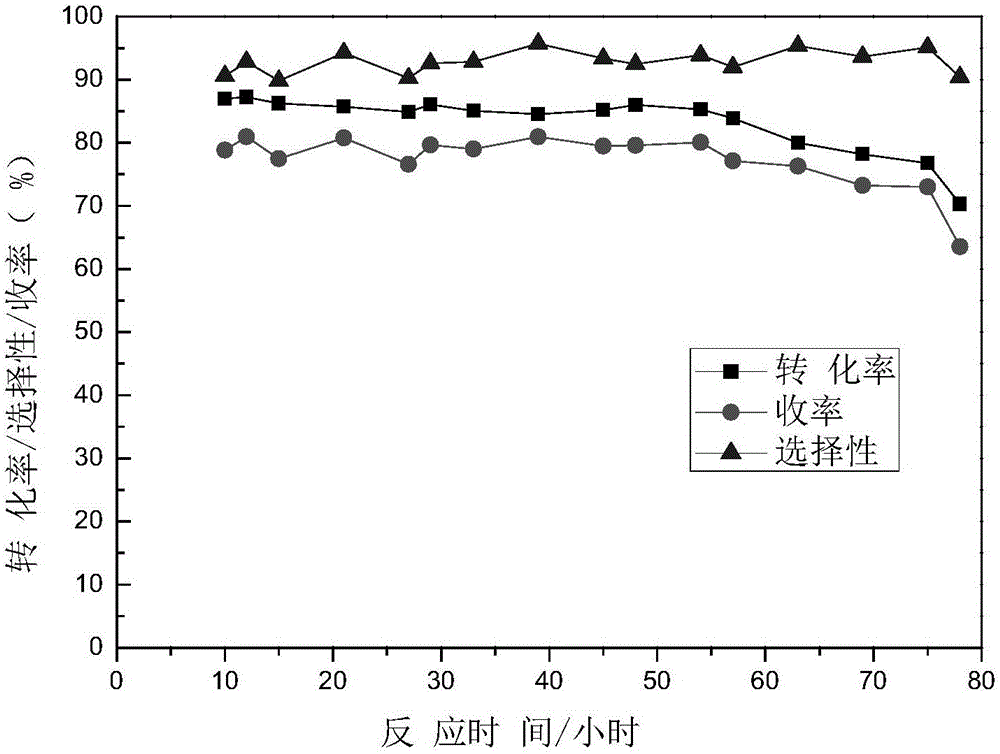
Method for preparing N-vinyl pyrrolidone in dehydration mode with N-hydroxyethyl pyrrolidone
A technology of hydroxyethylpyrrolidone and vinylpyrrolidone, which is applied in the field of preparing N-vinylpyrrolidone, and can solve problems such as increasing the market competitiveness of the dehydration method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0102] Embodiment 1: Preparation of N-vinylpyrrolidone
[0103] The implementation steps of this embodiment are as follows:
[0104] A. Preparation of catalyst
[0105] Prepare according to the catalyst preparation method described in Example 16 of US5625076 specification. Dissolve 10.7g of cesium nitrate in 150ml of deionized water at 90°C, add 30g of white carbon black, stir evenly, and evaporate the solution to dryness by a rotary evaporator. Then the evaporated sample was calcined at 550 °C for 2 hours to obtain the Cs / SiO 2 catalyst.
[0106]B, preparation of N-vinylpyrrolidone
[0107] The acidic component CO 2 Mix with N-hydroxyethylpyrrolidone vapor and inert gas N 2 Mix and heat to the reaction temperature to obtain a gaseous raw material mixture, the specific composition of the gaseous raw material mixture is 0.05CO 2 +0.05NHP+0.9N 2 ;Then,
[0108] Let the raw material mixture be at a temperature of 340°C, a pressure of 0.1MPa and a gas phase space velocity...
Embodiment 2
[0111] Embodiment 2: Preparation of N-vinylpyrrolidone
[0112] The implementation steps of this embodiment are as follows:
[0113] A. Preparation of catalyst
[0114] The catalyst prepared in Example 1 was used.
[0115] B, preparation of N-vinylpyrrolidone
[0116] The acidic component CO 2 Mix with N-hydroxyethylpyrrolidone vapor and water vapor, heat to the reaction temperature to obtain a gaseous raw material mixture, the specific composition of the gaseous raw material mixture is 0.9CO 2 +0.05NHP+0.05H 2 O; then,
[0117] Let the raw material mixture be at a temperature of 350°C, a pressure of 0.1MPa and a gas phase space velocity of 2000h -1 Under the conditions of the Cs / SiO loaded with the preparation of Example 1 2 The fixed-bed reactor of the catalyst, after 10 hours of reaction to reach stability, collect the N-vinylpyrrolidone product discharged from the fixed-bed reactor.
[0118] Using the same analysis method as in Example 1, the N-vinylpyrrolidone pro...
Embodiment 3
[0119] Embodiment 3: Preparation of N-vinylpyrrolidone
[0120] The implementation steps of this embodiment are as follows:
[0121] A. Preparation of catalyst
[0122] The catalyst in Example 1 was used.
[0123] B, preparation of N-vinylpyrrolidone
[0124] The acidic component CO 2 Mix with N-hydroxyethylpyrrolidone vapor and inert gas N 2 Mix and heat to the reaction temperature to obtain a gaseous raw material mixture, the specific composition of the gaseous raw material mixture is 0.3CO 2 +0.4NHP+0.3N 2 ;Then,
[0125] Let the raw material mixture be at a temperature of 370°C, a pressure of 0.1MPa and a gas phase space velocity of 2000h -1 Under the conditions of the Cs / SiO loaded with the preparation of Example 1 2 The fixed-bed reactor of the catalyst, after 10 hours of reaction to reach stability, collect the N-vinylpyrrolidone product discharged from the fixed-bed reactor.
[0126] Using the same analysis method as in Example 1, the N-vinylpyrrolidone produc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com