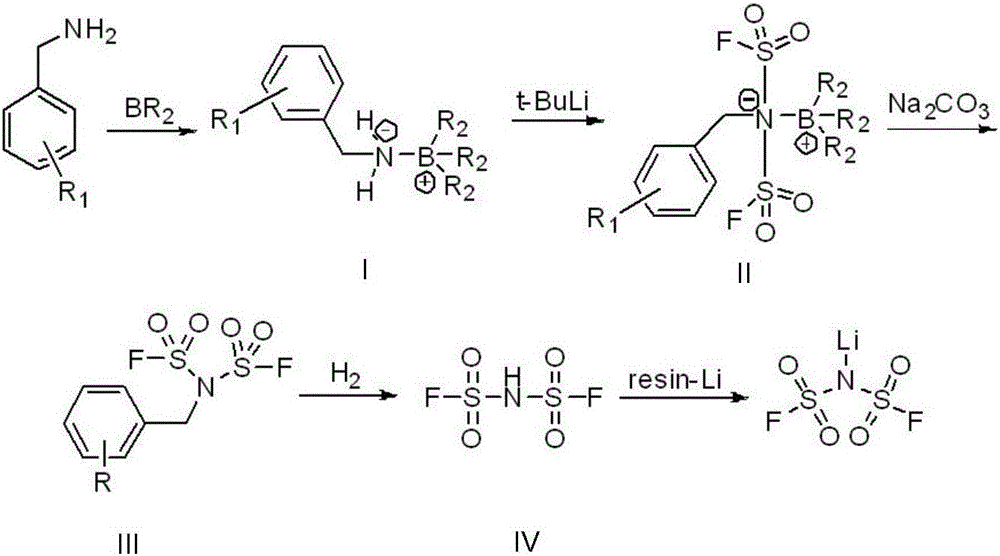
A preparing method of lithium bis(fluorosulfonyl)imide
A technology of bisfluorosulfonimide lithium salt and sulfonyl is applied in the field of preparation of bisfluorosulfonimide lithium salt, and can solve the problems of easy explosion safety, flammability and explosion, hidden danger and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Step a, weigh 10.7g of benzylamine, add it to a three-neck flask with a constant pressure dropping funnel and a thermometer, dissolve it in 100ml of tetrahydrofuran, and slowly add 1M BH dropwise after the temperature drops to 0°C 3 - 10.2ml of THF, the dropping time is 20min, the temperature of the whole dropping reaction is kept at 0°C-5°C, after the dropwise addition, directly distill under reduced pressure to obtain 12g of the intermediate.
[0028] Step b, add it into a three-neck flask with a constant pressure dropping funnel and a thermometer, dissolve it in 500ml of tetrahydrofuran, and slowly add 141ml of tert-butyl lithium dropwise after the temperature drops to -78°C. The dropping time is 1h, and the whole dropwise The reaction temperature is kept at -75°C to -78°C, and after the dropwise addition is completed, the reaction is continued at -75°C to -78°C for 1 hour. After the reaction is completed, 17.4ml of fluorosulfuryl chloride is slowly added dropwise, an...
Embodiment 2~3
[0032] The reaction steps are the same as in Example 1, except that the starting material in step a is replaced by 4-methylbenzylamine and 3,5-bismethylbenzylamine respectively, and the finally obtained aromatic methyl The yields of bisfluorosulfonimide were 78% and 74%, respectively.
Embodiment 4~5
[0034] The reaction steps are substantially the same as in Example 1, except that the BH in step a 3 -THF was replaced by trimethoxyboron and trihexyloxyboron respectively, and the yields of the finally obtained aromatic methylboronic acid were 88% and 83%, respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com