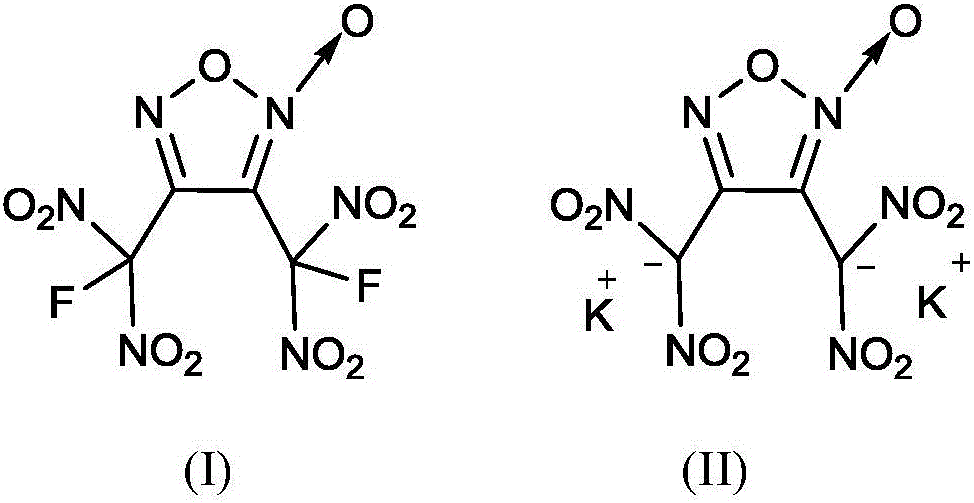
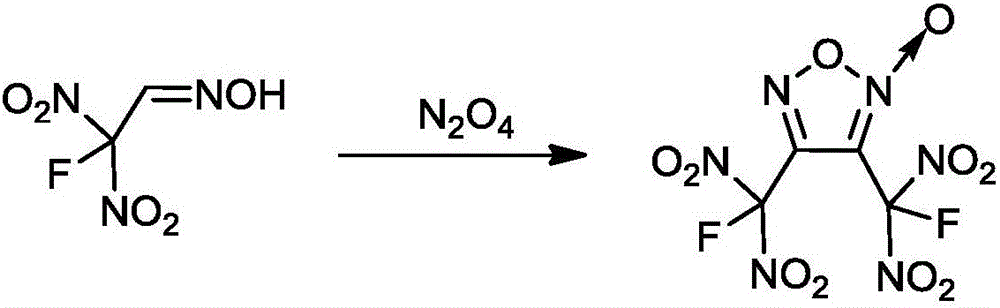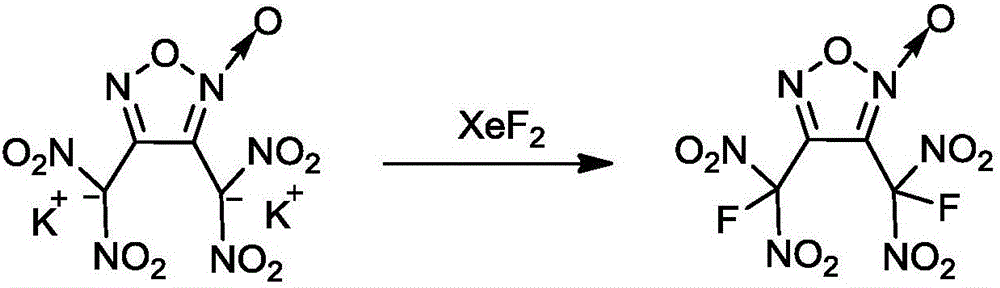Method for synthesizing 3, 4-di (fluorine geminal dinitro) dinitrofurzananofuroxan
A technology of fluoro-gem-dinitro and furoxan, which is applied in the field of synthesis of 3,4-dioxyfurazan, can solve the problems of cumbersome processing and low yield, and achieve the effect of simple post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Under stirring, add 0.74g (2mmol) of 3,4-bis(dinitromethyl)furazan dipotassium salt into 15mL of acetonitrile, then add 2.03g (12mmol) of xenon difluoride, and react at a temperature of 25°C 96h, concentrate the reaction solution, pour the obtained concentrated solution into water, use dichloromethane (10mL×3) to extract the reaction solution, combine the extracts, wash with water, dry, and distill under reduced pressure to obtain a colorless oily liquid 3,4- Bis(fluoro-gem-dinitro)furoxan 0.39g, yield 59.0%, purity 98.6%;
[0019] Structure Identification:
[0020] Infrared Spectrum: IR(KBr,cm -1 )ν: 1662, 1611, 1484, 1360, 1342, 1300, 1260, 1212, 1158, 989, 926, 835, 793, 705, 632;
[0021] NMR spectrum: 13 CNMR (CDCl 3 ,125MHz), δ:141.70,114.62,113.91,103.84; 19 F NMR (DMSO-d 6 ,470.5MHz), δ:-93.87,-100.16;
[0022] Elemental Analysis: Structural Formula C 4 N 6 o 10 f 2
[0023] Theoretical value: C 14.56, N 25.46
[0024] Measured value: C 14.48, N 25....
Embodiment 2
[0027] Under stirring, add 0.74g (2mmol) of 3,4-bis(dinitromethyl)furazan dipotassium salt into 15mL of acetonitrile, then add 2.03g (12mmol) of xenon difluoride, and react at a temperature of 20°C 100h, concentrate the reaction solution, pour the obtained concentrated solution into water, extract the reaction solution with dichloromethane (10mL×3), combine the extracts, wash with water, dry, and distill under reduced pressure to obtain a colorless oily liquid 3,4- Bis(fluoro-gem-dinitro)furoxan 0.34 g, yield 51.4%, purity 98.5%.
Embodiment 3
[0029] Under stirring, add 0.74g (2mmol) of 3,4-bis(dinitromethyl)furazan dipotassium salt into 15mL of acetonitrile, then add 2.03g (12mmol) of xenon difluoride, and react at a temperature of 15°C 80h, concentrate the reaction solution, pour the obtained concentrated solution into water, extract the reaction solution with dichloromethane (10mL×3), combine the extracts, wash with water, dry, and distill under reduced pressure to obtain a colorless oily liquid 3,4- Bis(fluorogem-dinitro)furoxan 0.22 g, yield 33.0%, purity 97.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com