Soluble isomerized anthracene-containing polyimide with information storage function
An anthracene polyimide and information storage technology, applied in the field of organic information storage materials, achieves the effects of stable storage performance, low switching voltage, and excellent electrical bistable information storage performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
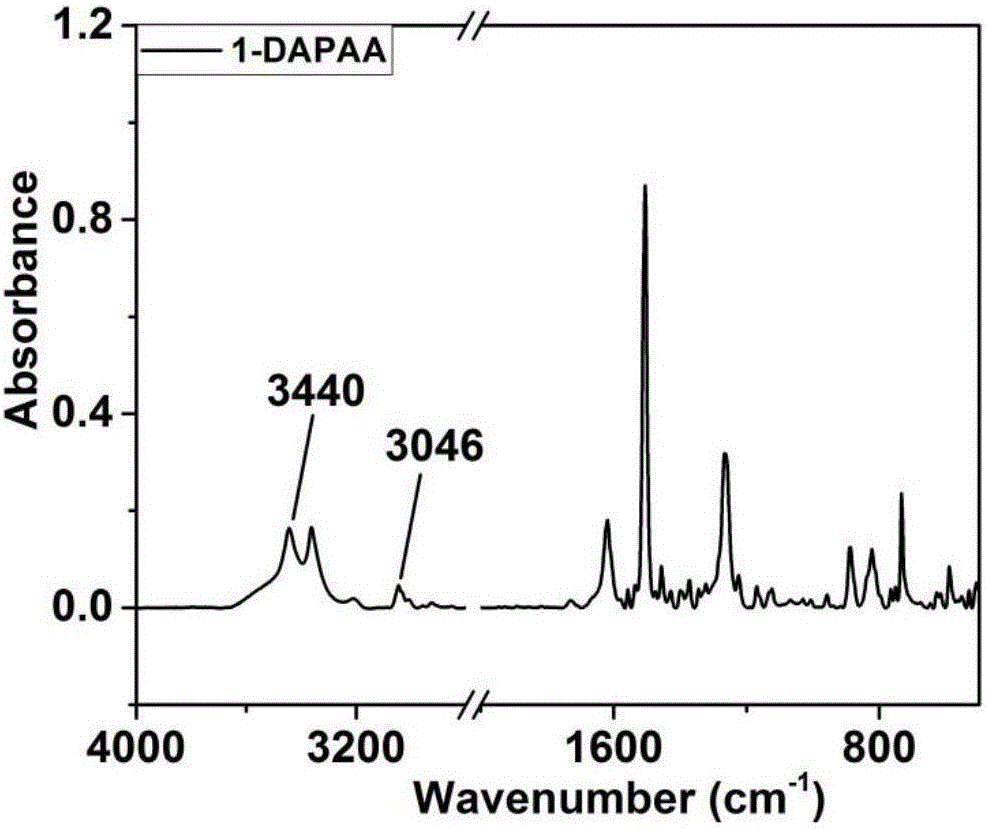
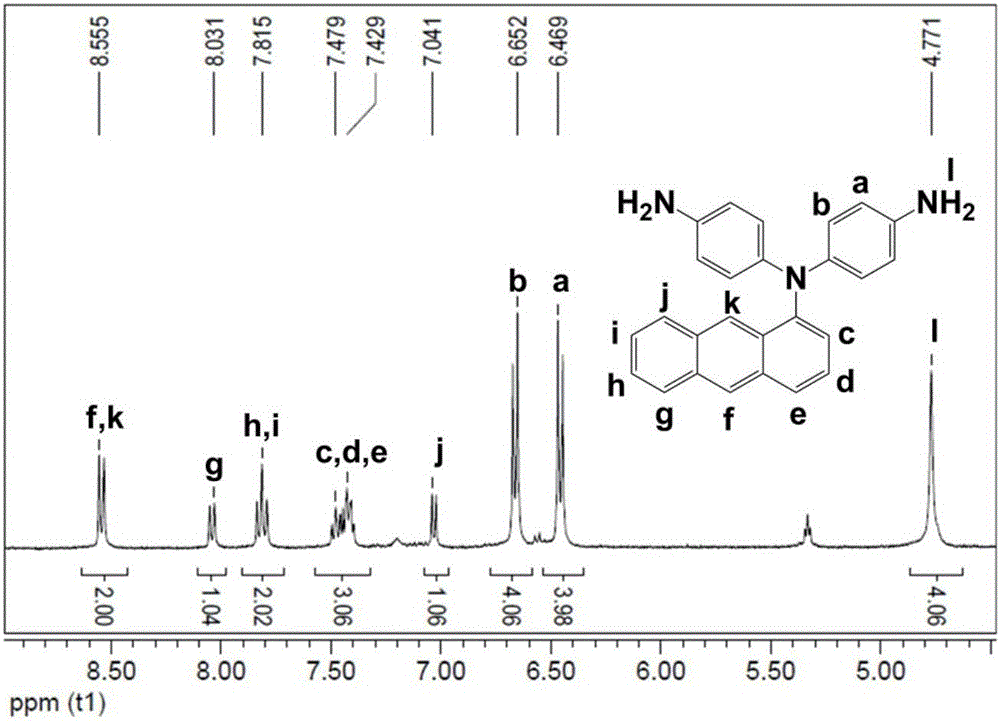
[0039] Preparation of N,N-bis(4-aminophenyl)-1-aminoanthracene:
[0040] Under the protection of nitrogen atmosphere, add 1.9324g (10mmol) of 1-aminoanthracene, 3.2453g (23mmol) of p-nitrofluorobenzene, 3.45g of potassium carbonate, and 30ml of dimethyl sulfoxide into a 100ml three-necked flask, seal the system and turn on the magnetic force Stirring and heating, the temperature is set to 150°C. Stirring and heating were stopped after 48 hours of magnetic stirring. The solution in the three-necked flask was poured into ice methanol to obtain a bright yellow solid, and the obtained solid was washed with deionized water and ice methanol several times successively. After filtration, the solid was dried in a vacuum oven for 12 hours to obtain a bright yellow powder. Take 1.8774g of the bright yellow powder and put it into a 100ml three-necked bottle, then add 0.5315g of palladium carbon catalyst and 50ml of ethanol successively, turn on magnetic stirring and heating, set the temp...
Embodiment 2
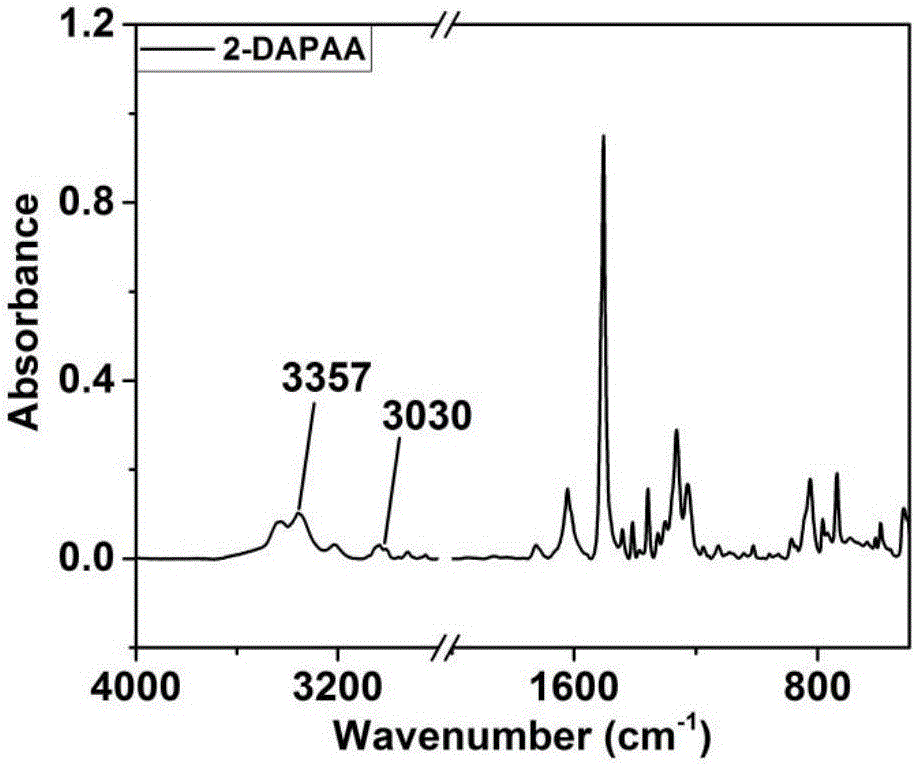
[0044] Preparation of N,N-bis(4-aminophenyl)-2-aminoanthracene:
[0045] Under the protection of nitrogen atmosphere, add 1.9324g (10mmol) of 2-aminoanthracene, 3.2453g (23mmol) of p-nitrofluorobenzene, 3.44g of potassium carbonate, and 30ml of dimethyl sulfoxide into a 100ml three-necked flask, seal the system and turn on the magnetic force Stirring and heating, the temperature is set to 150°C. Stirring and heating were stopped after 48 hours of magnetic stirring. The solution in the three-necked flask was poured into ice methanol to obtain a bright yellow solid, and the obtained solid was washed with deionized water and ice methanol several times successively. After filtration, the solid was dried in a vacuum oven for 12 hours to obtain a bright yellow powder. Take 1.5019g of the bright yellow powder and put it into a 100ml three-neck bottle, then add 0.4260g of palladium carbon catalyst and 40ml of ethanol successively, turn on magnetic stirring and heating, set the temper...
Embodiment 3
[0049] Preparation of N,N-bis(4-aminophenyl)-9-aminoanthracene:
[0050] Under the protection of nitrogen atmosphere, add 1.9324g (10mmol) of 1-aminoanthracene, 3.2453g (23mmol) of p-nitrofluorobenzene, 3.44g of potassium carbonate, and 30ml of dimethyl sulfoxide into a 100ml three-necked flask, seal the system and turn on the magnetic force Stirring and heating, the temperature is set to 150°C. Stirring and heating were stopped after 48 hours of magnetic stirring. The solution in the three-necked flask was poured into ice methanol to obtain a bright yellow solid, and the obtained solid was washed with deionized water and ice methanol several times successively. After filtration, the solid was dried in a vacuum oven for 12 hours to obtain a bright yellow powder. Take 1.1264g of the bright yellow powder and put it into a 100ml three-neck flask, then add 0.3195g of palladium carbon catalyst and 30ml of ethanol successively, turn on magnetic stirring and heating, set the tempera...
PUM
| Property | Measurement | Unit |
|---|---|---|
| polydispersity index | aaaaa | aaaaa |
| polydispersity index | aaaaa | aaaaa |
| polydispersity index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com