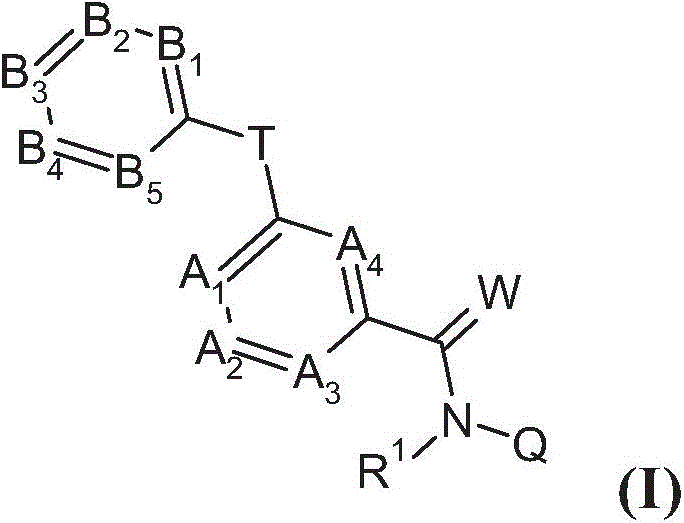
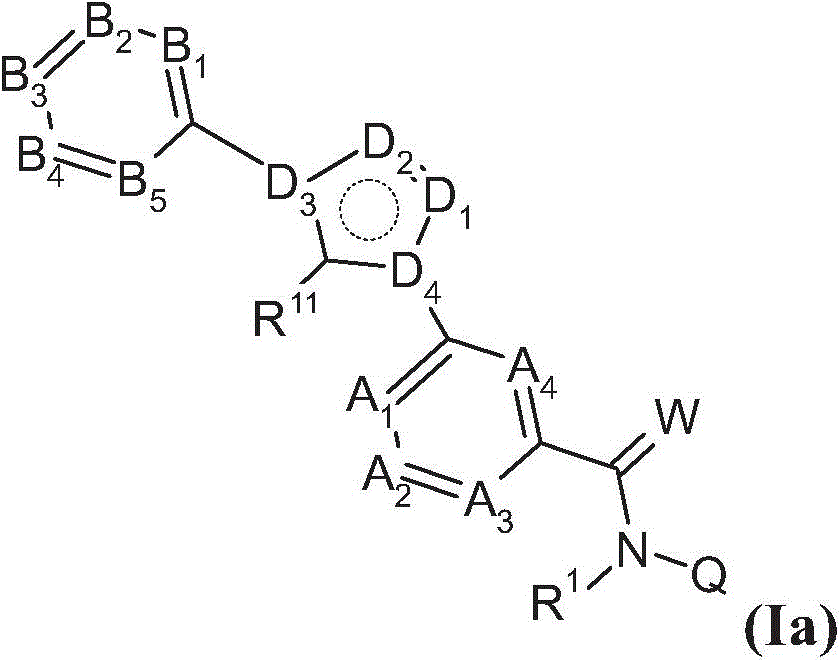
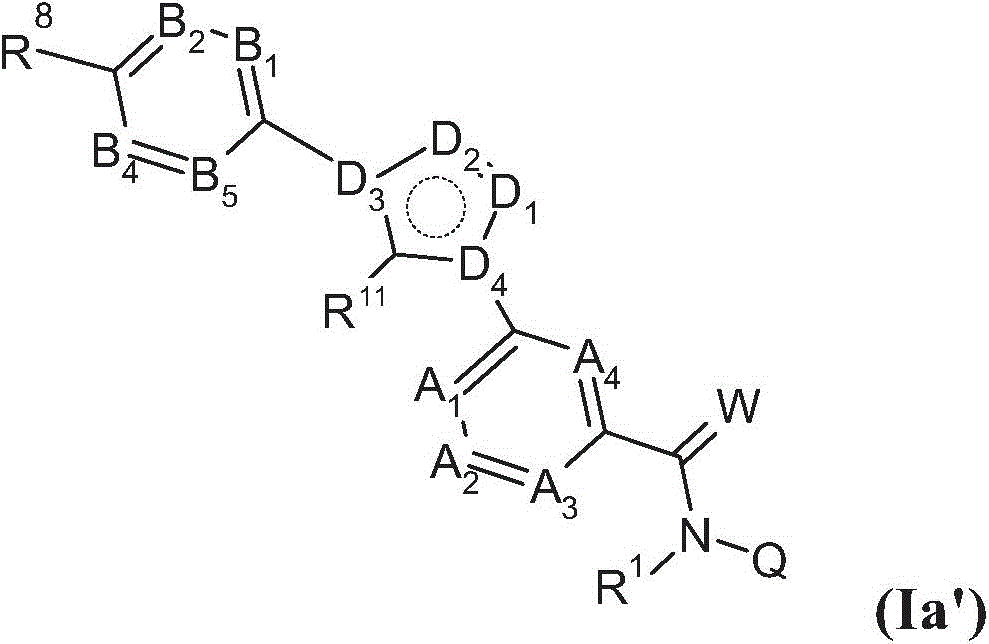
Substituted benzamides for treating arthropodes
A technology of compounds and oxides, applied in the field of arachnids and nematodes, insects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0138] The definition of collective terms also applies to these collective terms for composite substituents, unless defined otherwise. Example: C LL -C UL - The definition of alkyl also applies to C LL -C UL -Alkyl as compound substituent such as (C LL -C UL )-Cycloalkyl-(C LL -C UL )-part of an alkyl group.
[0139] It will be appreciated by those skilled in the art that the examples cited in this application should not be considered in a limiting manner, but merely to describe some embodiments in detail.
[0140] In the definitions of symbols given in the above formulas, general designations generally representing the following substituents are used:
[0141] Halogen relates to elements of the seventh main group, preferably fluorine, chlorine, bromine and iodine, more preferably fluorine, chlorine and bromine, and even more preferably fluorine and chlorine.
[0142] Examples of heteroatoms are N, O, S, P, B, Si. Preferably, the term "heteroatom" relates to N, S and...
Embodiment I-T2-1
[1295]
[1296] 710 mg (2.24 mmol) of 1-[2,6-dimethyl-4-[1,2,2,2-tetrafluoro-1-(trifluoromethyl)ethyl]phenyl]ethanone was added to 401 mg (3.36 mmol) of N,N-dimethylformamide dimethyl acetal, and the mixture was heated to reflux for 5 hours. For work-up, the mixture was cooled slightly and all volatile components were evaporated off with a rotary evaporator under reduced pressure. The residue was chromatographed using a column containing 40 g of silica gel with a cyclohexane / ethyl acetate gradient from 90:10 to 50:50 (v / v). 675 mg of 3-(dimethylamino)-1-[2,6-dimethyl-4-[1,2,2,2-tetrafluoro-1-(tetrafluoromethyl)ethyl]phenyl were obtained ] prop-2-en-1-one.
[1297]
[1298] 1.2g (3.23mmol) of 3-(dimethylamino)-1-[2,6-dimethyl-4-[1,2,2,2-tetrafluoro-1-(tetrafluoromethyl) Ethyl]phenyl]prop-2-en-1-one was added to 15.5 ml of ethanol, and 170 mg (3.39 mmol) of hydrazine hydrate and 192 mg (3.2 mmol) of glacial acetic acid were added. The resulting mixture was stirred at r...
Embodiment I-T3-1
[1316]
[1317] The preparation of the precursor [2,6-dimethyl-4-[1,2,2,2-tetrafluoro-1-(trifluoromethyl)ethyl]phenyl]hydrazine is described in the literature (US 2003 / 187233).
[1318]
[1319] First, 3.41 g (11.2 mmol) of [2,6-dimethyl-4-[1,2,2,2-tetrafluoro-1-(trifluoromethyl)ethyl] in 13 ml of ethanol Phenyl]hydrazine (free base) was charged into a 25ml flask. Then 1.84 g (11.2 mmol) of tetramethoxypropane were added, followed by 0.55 g (5.6 mmol) of 96% sulfuric acid. The reaction mixture was heated to reflux for 2h. Ethanol was distilled off on a rotary evaporator under reduced pressure. The residue was partitioned between ethyl acetate and saturated aqueous sodium bicarbonate. The organic phase was removed, dried over sodium sulfate and concentrated under reduced pressure on a rotary evaporator. The residue was distilled under reduced pressure in a Kugelrohr at 1 mbar and 150° C. to obtain 2.5 g of 1-[2,6-dimethyl-4-[1,2,2,2-tetrafluoro-1-(trifluoromethane ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com