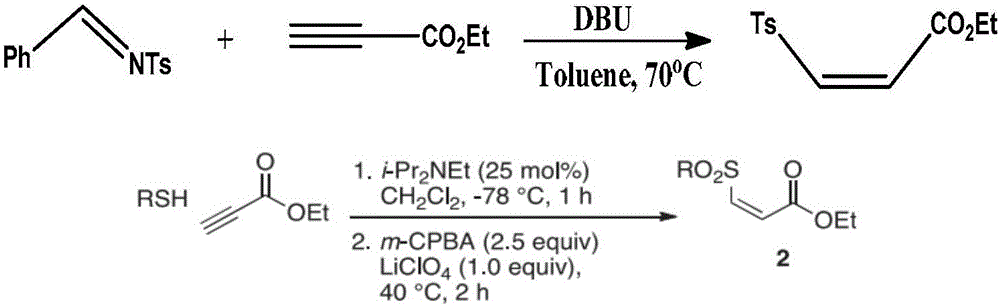
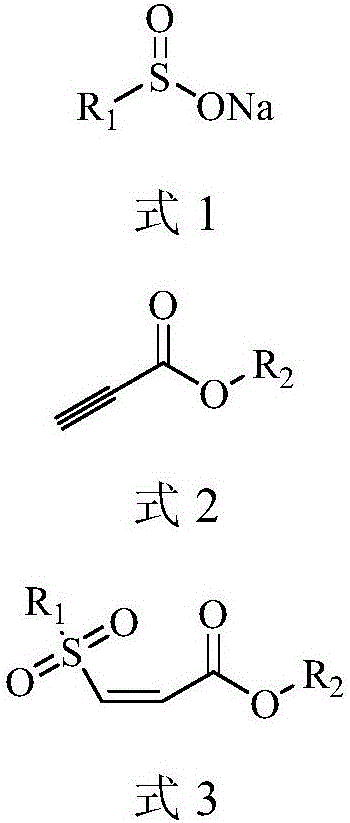
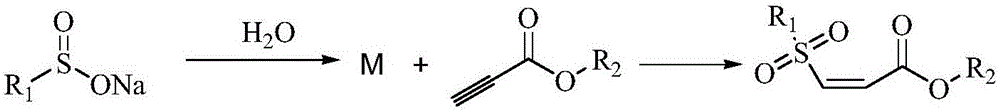
One-pot synthesis method of (Z)-type sulfonyl olefine acid ester compound
A technology of sulfonyl alkenoate and synthesis method, applied in the preparation of organic compounds, chemical instruments and methods, organic chemistry, etc., can solve the problems of low selectivity, narrow substrate range, low atom economy, etc. The effect of simplified process steps, mild reaction conditions and wide application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033]In a clean and dry 10 ml round bottom flask, add 49 mg of sodium p-toluene sulfinate and 74 mg of ethyl propiolate in sequence, use 2 ml of water as the reaction solvent, and stir at 70°C for 10 minutes. After the reaction was completed, it was extracted by adding ethyl acetate, and the upper organic phase was directly spin-dried and dissolved with a small amount of petroleum ether and ethyl acetate (30:1 by volume), and separated by a silica gel column to obtain 58.4 mg of a colorless liquid. Yield 92%.
[0034] 1 H NMR (400MHz, CDCl 3 )δ7.81(d, J=8.1Hz, 2H), 7.27(d, J=8.0Hz, 2H), 6.50–6.31(m, 2H), 4.29(q, J=7.1Hz, 2H), 2.39( s,3H),1.31(t,J=7.1Hz,3H); 13 CNMR (100MHz, CDCl 3 )δ164.03, 145.20, 136.50, 135.41, 131.42, 129.97, 128.28, 62.15, 21.69, 13.96.
Embodiment 2
[0036] In a clean and dry 10 ml round bottom flask, add 53 mg of sodium p-methoxyphenyl sulfinate and 74 mg of ethyl propiolate in sequence, and use 2 ml of water as the reaction solvent, and stir for 5 minutes at 50°C . After the reaction was completed, it was extracted by adding ethyl acetate, and the upper organic phase was directly spin-dried and dissolved with a small amount of petroleum ether and ethyl acetate (30:1 by volume), and separated by a short silica gel column to obtain 66.2 mg of colorless Liquid, 96% yield.
[0037] 1 H NMR (400MHz, CDCl 3 )δ7.92(d, J=8.7Hz, 2H), 7.05(d, J=8.7Hz, 2H), 6.532–6.40(m, 2H), 4.36(q, J=7.1Hz, 2H), 3.87( s,3H),1.38(t,J=7.1Hz,3H); 13 C NMR (100MHz, CDCl 3 )δ164.15, 164.12, 135.67, 130.88, 130.59, 114.57, 62.12, 55.72, 14.03.
Embodiment 3
[0039] In a clean and dry 10 ml round bottom flask, add 65 mg of sodium p-bromophenylsulfinate and 74 mg of ethyl propiolate in sequence, and use 2 ml of water as the reaction solvent, and stir at 45°C for 15 minutes. After the reaction, it was extracted by adding ethyl acetate, and the upper organic phase was directly spin-dried and dissolved with a small amount of petroleum ether and ethyl acetate (volume ratio of 30:1), separated by a short silica gel column to obtain 73.4 mg of white solid , yield 92%.
[0040] 1 H NMR (400MHz, CDCl 3 )δ7.88(d, J=8.4Hz, 2H), 7.72(d, J=8.4Hz, 2H), 6.52(s, 2H), 4.36(q, J=7.1Hz, 2H), 1.38(t, J = 7.2Hz, 2H); 13 C NMR (100MHz, CDCl 3 )δ163.77, 138.50, 135.01, 132.66, 132.55, 129.83, 129.54, 62.30, 13.98.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com