Withanolides useful for the treatment of neurodegenerative diseases
一种化合物、烯基的技术,应用在神经系统疾病、神经肌肉系统疾病、肌肉系统疾病等方向,能够解决半衰期短毒性等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0114] Preparation of compounds of formula (I)
[0115] The following reaction scheme shows a semi-synthetic method for the preparation of compounds of formula (Ia) using withaferin A as a starting material. More specifically, withaferin A (available from Sigma-Aldrich Canada) can be treated with one or more alkylating agents to alkylate the -OH group of withardin A.
[0116] Reaction formula
[0117]
[0118] This reaction can produce compounds that are monoalkylated at C-4 or C-27, as well as mixtures of dialkylated WAs at both C-4 and C-27. The alkylated compound can be isolated and isolated by methods known in the art. When R 1 and R 3 At the same time, a single alkylation step can be performed. When R 1 and R 3 At different times, two alkylation steps can be carried out. For example, the alkylating agent R can be used 1 -X and R 3 -X (X is a leaving group) undergoes a stepwise reaction to independently alkylate the hydroxyl group of withaferin A.
[0119] Se...
Embodiment 1
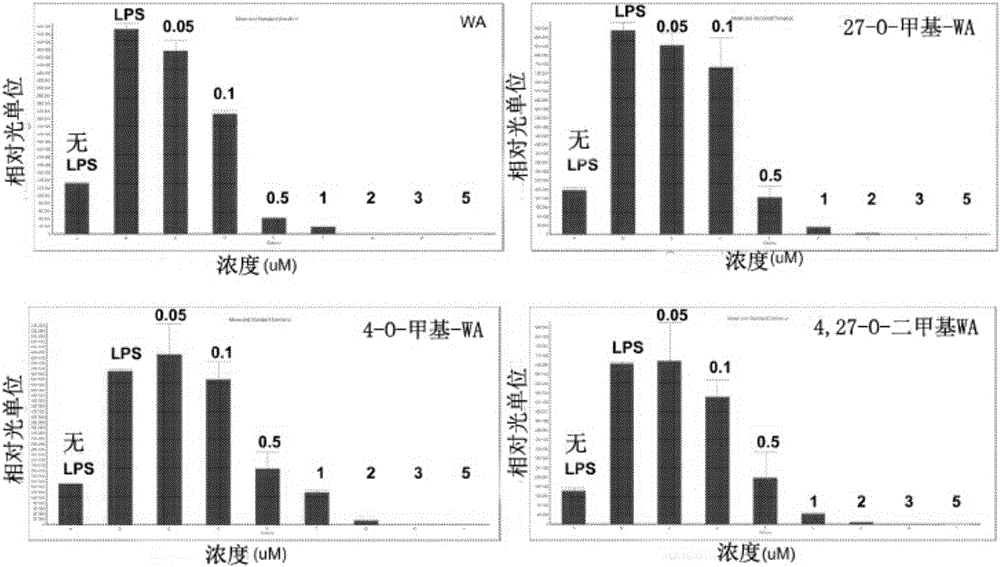
[0127] Preparation of WA methyl ether analogs from WA
[0128]
[0129] 1 Withaferin A:R 1 = R 2 =H
[0130] 2 27-O-Methyl withaferin A:R 1 =CH 3 , R 2 =H
[0131] 3 4-O-Methyl withaferin A:R 1 =H,R 2 =CH 3
[0132] 4 4,27-O-Dimethyl withafarain A:R 1 = R 2 =CH 3
[0133] preparation
[0134] 150 mg of withaferin A (available from Sigma-Aldrich Canada) was treated with sodium hydride dissolved in methyl iodide (WA 1). This reaction produces a mixture of monomethylated compound and dimethylated WA. The reaction mixture was filtered to remove excess sodium hydride and sodium iodide. The filtrate was dried and the residue was redissolved in dichloromethane. The resulting solution was chromatographed on a silica gel column. combined with a single methyl ether ( 2 and 3 ) and dimethyl ether ( 4 ) and the compound was isolated by reverse phase chromatography.
[0135] The pure fractions of each compound were combined, concentrated, extracted into dichlo...
Embodiment 2
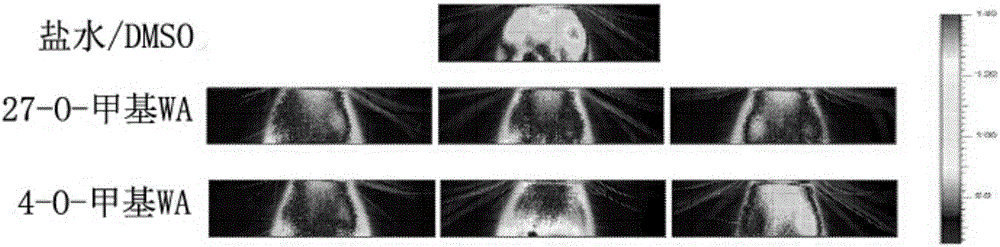
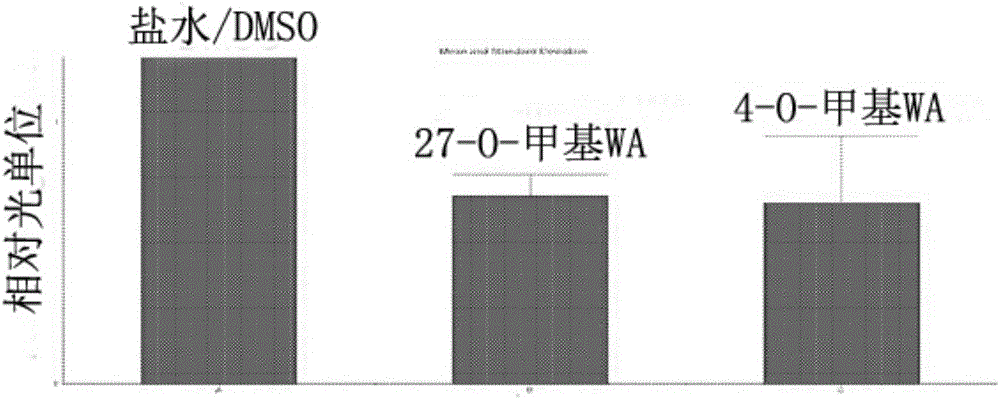
[0139] Brain bioluminescence of GFAP-luciferase mice exposed to LPS.
[0140] Therapeutic activity of two novel withanolides was tested in vivo using a transgenic mouse model. The ability of withanolalide to inhibit inflammation-associated astrocyte glialization induced by exposure to lipopolysaccharide (LPS) was assessed using transgenic GFAP luciferase mice generated in the laboratory of Dr. J.P. Julienwere. In vivo bioluminescence imaging was performed to assess the inflammatory response in the cranium. The decrease in the bioluminescent signal in the assessed area compared to the control (saline) group indicated that withanolide crossed the blood-brain barrier and subsequently inhibited gliosis.
[0141] Dilute withabalide [4-O-methyl withafanaline A (4-O-methyl WA) and 27-O-methyl withafanaline A (27-O-methyl WA)] in 100% In dimethyl sulfoxide (DMSO), the final concentration was 2 mg / ml. The in vivo efficacy of these analogs was tested using GFAP-luc transgenic mice. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com