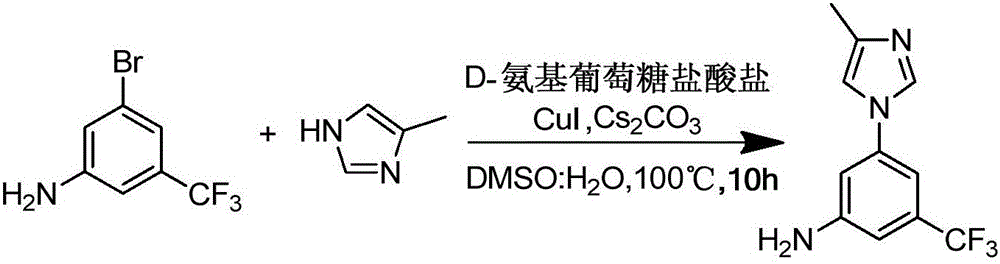
Preparation method of nilotinib intermediate-3-(4-methyl-1H-imidazol-1-yl)-5-(trifluoromethyl)aniline
A technology of trifluoromethylaniline and trifluoromethyl, which is applied in the field of preparation of nilotinib intermediate 3--5-aniline, can solve the problems of low reaction yield, large environmental pollution, and unsuitability for industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] In the there-necked flask, add 24g (0.1mol) 3-bromo-5-trifluoromethylaniline, 8.2g (0.1mol) 4-methylimidazole, 65.2g (0.2mol) Cs 2 CO 3 , 1.9g (0.01mol) CuI, 2.2g (0.01mol) D-glucosamine hydrochloride, then add 50mL DMSO and 50mL water, stir to dissolve, control the reaction temperature at 100°C, and continue the reaction for 10 hours. Add 100mL ethyl acetate after completion of the reaction, get the supernatant after centrifugation, concentrate and dry to obtain 3-(4-methyl-1H-imidazol-1-yl)-5-(trifluoromethyl)aniline, the HPLC purity is 98.53%, the yield is 95.25%.
Embodiment 2
[0022] In the reaction vessel, add 24g (0.1mol) 3-bromo-5-trifluoromethylaniline, 8.2g (0.1mol) 4-methylimidazole, 58.7g (0.18mol) Cs 2 CO 3 , 0.95g (0.005mol) CuI, 1.1g (0.005mol) D-glucosamine hydrochloride, then add 60mL DMSO and 80mL water, stir to dissolve it, control the reaction temperature to 90°C, and continue to react for 12 hours. After completion, 120 mL of ethyl acetate was added, and the supernatant was taken after centrifugation, concentrated and dried to obtain 3-(4-methyl-1H-imidazol-1-yl)-5-(trifluoromethyl)aniline. The HPLC purity was 98.66%, and the yield was 96.18%.
Embodiment 3
[0024] In the reaction vessel, add 24g (0.1mol) 3-bromo-5-trifluoromethylaniline, 8.2g (0.1mol) 4-methylimidazole, 71.7g (0.22mol) Cs 2 CO 3 , 3.8g (0.02mol) CuI, 4.4g (0.02mol) D-glucosamine hydrochloride, then add 80mL DMSO and 60mL water, stir to make it dissolve, control the reaction temperature to be 110°C, and continue to react for 8 hours. After completion, add 160 mL of ethyl acetate, take the supernatant after centrifugation, concentrate and dry to obtain 3-(4-methyl-1H-imidazol-1-yl)-5-(trifluoromethyl)aniline. The HPLC purity was 98.09%, and the yield was 95.77%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com