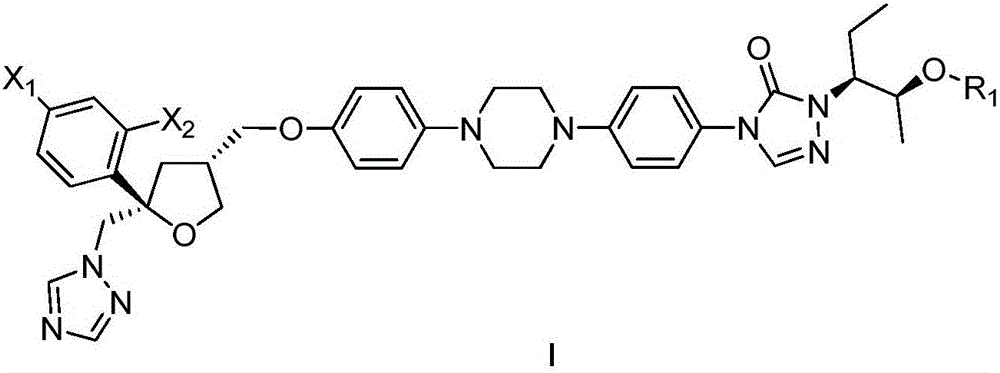
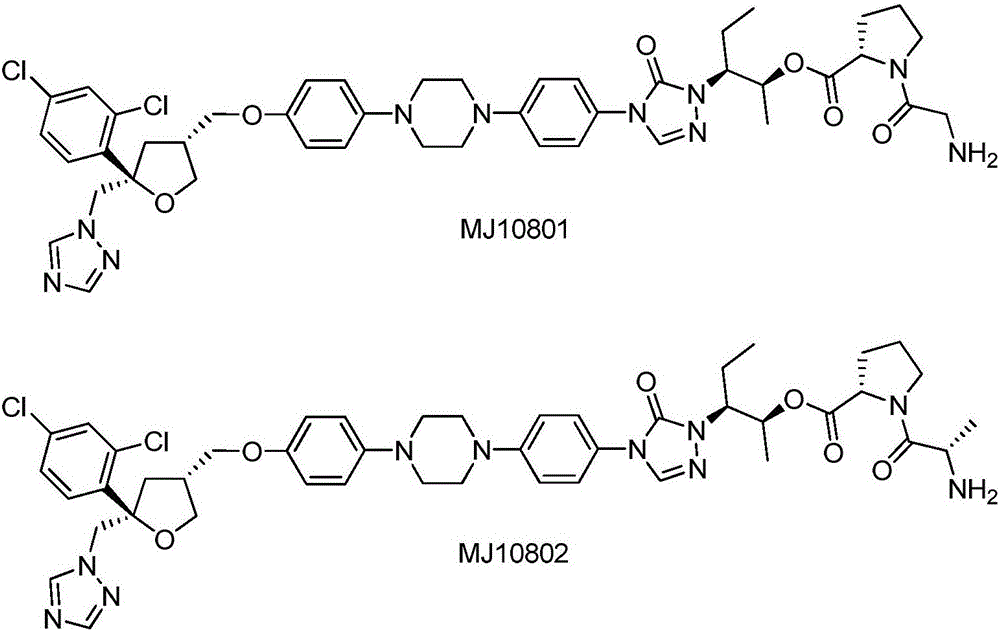
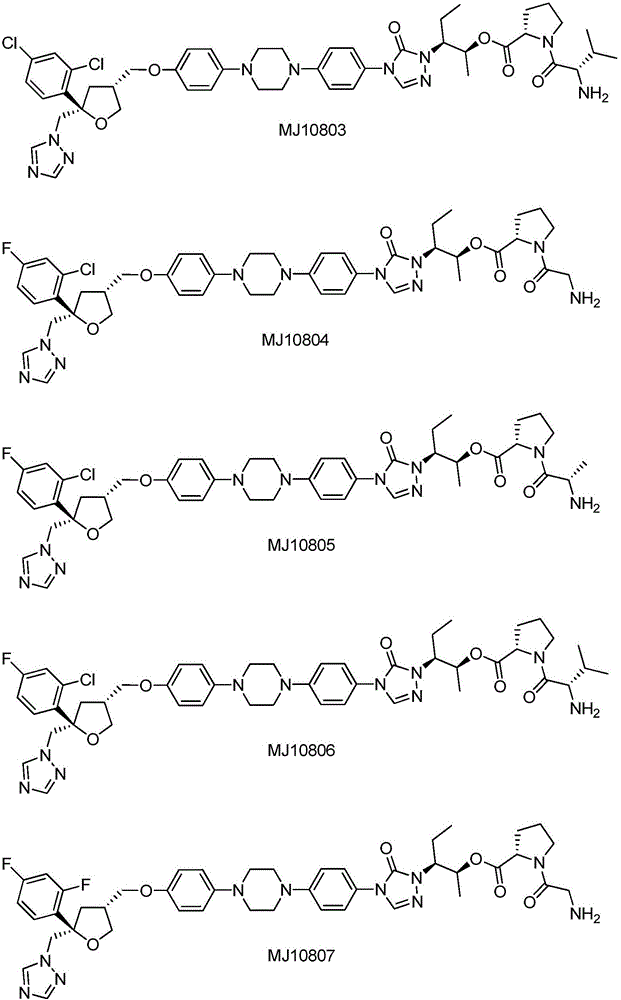
Substituted tetrahydrofuran water-soluble derivatives and application thereof
A hydrate and solvate technology, applied in the field of medicinal chemistry, can solve problems such as poor water solubility, pain, and patient inconvenience
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1: the synthesis of MJ10821
[0049] (1) Synthesis of intermediate M21
[0050]
[0051] Dissolve Cbz-Val-Ala-OH (322mg, 1mmol) in 3ml N,N-dimethylformamide (DMF), cool down to -5°C, add DIC (63.1mg, 0.5mmol) under stirring, and react at room temperature 30min, continue to cool down to -5°C, add II3 (350mg, 0.5mmol) in 2ml DMF solution, triethylamine (60.7mg, 0.6mmol), catalytic amount of 4-dimethylaminopyridine (DMAP), and react at room temperature for 4h, After the reaction was completed, it was poured into 15ml of water, extracted 3 times with 15ml of ethyl acetate, the organic layers were combined, dried over anhydrous sodium sulfate, and the solvent was spin-dried to obtain a yellow solid, which was separated by silica gel column chromatography to obtain 281 mg of off-white powder (M21). Yield 56%. MS(m / z): 1005.5[M+1] + .
[0052] (2) Synthesis of MJ10821
[0053]
[0054] Dissolve M21 (251mg, 0.25mmol) in 3ml of methanol, add 200mg of 5% Pd / ...
Embodiment 2
[0055] Embodiment 2: the synthesis of MJ10807
[0056] Refer to the synthesis of MJ10821, the difference is that Cbz-Gly-Pro-OH and II3 are selected as starting materials, and after condensation and deprotection, MJ10807 is finally obtained. MS(m / z): 855.4[M+1] + .
Embodiment 3
[0057] Embodiment 3: the synthesis of MJ10808
[0058] Refer to the synthesis of MJ10821, the difference is that Cbz-Ala-Pro-OH and II3 are selected as starting materials, and after condensation and deprotection, MJ10808 is finally obtained. MS(m / z): 869.4[M+1] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com