N-acyl-1,2-cyclo-nitrogen-p-menthane, as well as preparation method and herbicidal activity application thereof
A technology of ring nitrogen paramentane and acyl group, which is applied in the field of N-acyl-1,2-cyclic nitrogen paramentane and its preparation, can solve the problems of no discovery, inapplicability and the like, and achieves low environmental pollution, low toxicity, Easy to apply effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Add 19.9 mL of 10% HCl aqueous solution, 5.08 g of N,N'-acyl-1,8-p-mentanediamine (such as N, N'-Diacetyl-1,8-p-mentanediamine, N,N'-dibutyryl-1,8-p-mentanediamine, N,N'-dipentanoyl-1,8-p Menthanediamine, N,N'-dicaproyl-1,8-p-mentanediamine, N,N'-dibenzoyl-1,8-p-menthanediamine and N,N'-di Phenylacetyl-1,8-p-menthanediamine, etc.), heated to reflux for 26h. After the reaction was completed, it was left to stand, and the reaction solution was separated into layers. After the pH of the lower layer solution was adjusted to 8-11, the solution was layered, and the upper layer solution was dried and subjected to silica gel column chromatography to obtain the corresponding N-acyl-1,2-cycloaza-p-menthane product.
Embodiment 2
[0048] In the 100mL four-neck flask equipped with a thermometer, a condenser tube and a mechanical stirrer, add 20mL concentration of 10% HCl aqueous solution, 2g N-acyl-3-p-mentene-1-amine (such as N-acetyl-3- p-menten-1-amine, N-butyryl-3-p-menten-1-amine, N-pentanoyl-3-p-menten-1-amine, N-hexanoyl-3-p-menten-1 -amine, N-benzoyl-3-p-menthene-1-amine and N-phenylacetyl-3-p-menthen-1-amine, etc.), heated to reflux for 24h. After the reaction was completed, it was left to stand, and the reaction solution was separated into layers. After the pH of the lower layer solution was adjusted to 8-11, the solution was layered, and the upper layer solution was dried and subjected to silica gel column chromatography to obtain the corresponding N-acyl-1,2-cycloaza-p-menthane product.
Embodiment 3
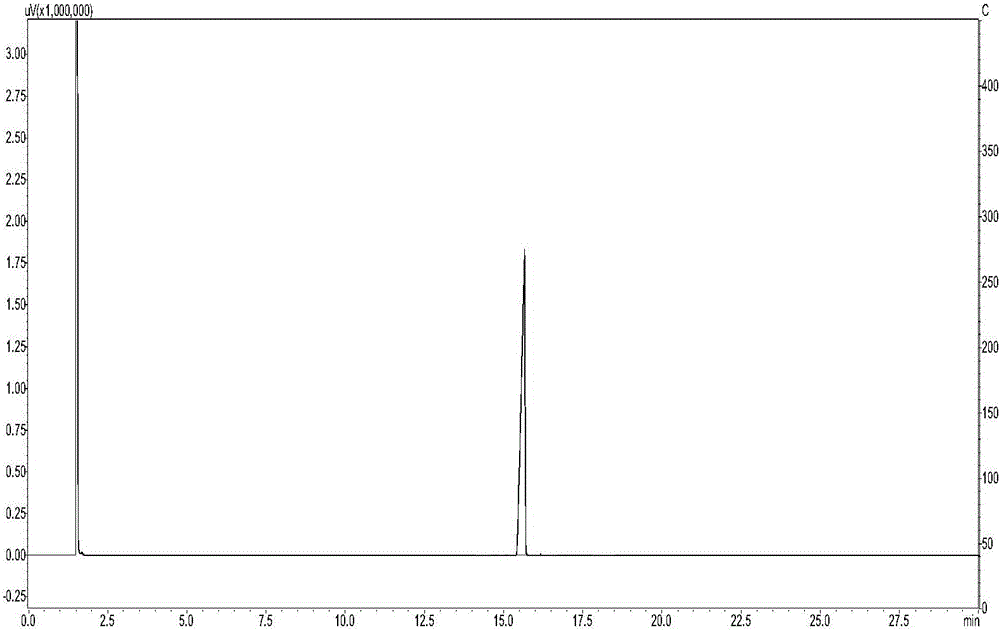
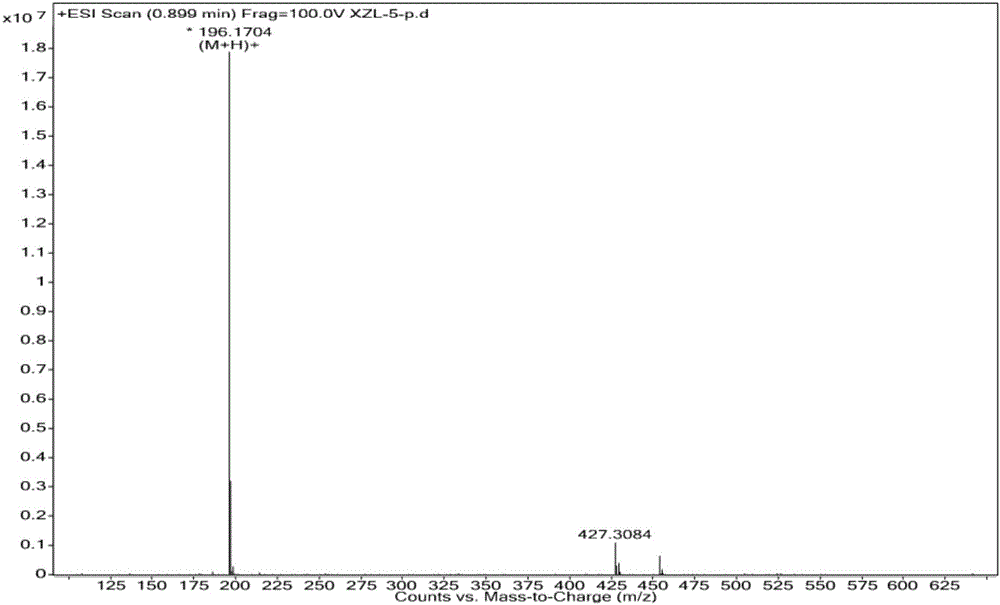
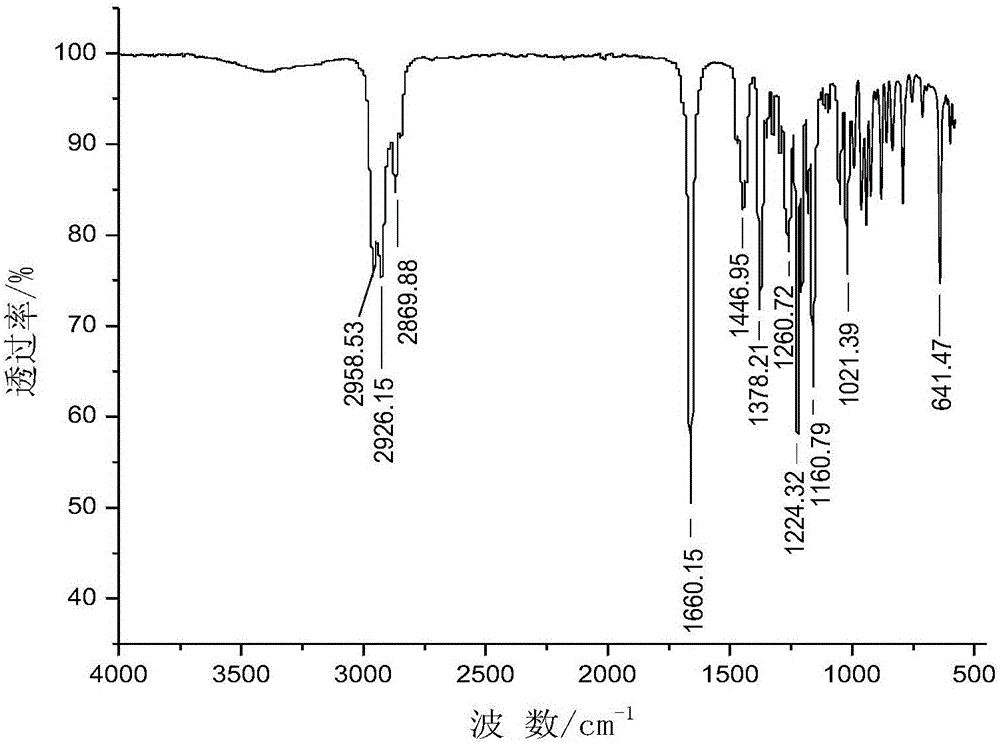
[0050] Add 198.9mL of 10wt.% HCl aqueous solution and 50.8g of N,N'-diacetyl-1,8-p-mentanediamine to a 500mL four-necked flask equipped with a thermometer, a condenser and a mechanical stirrer, Start stirring, heating to reflux for 30h. After the reaction, it was left standing, and the reaction solution was separated into layers, the upper layer was a yellow liquid, and the lower layer was an aqueous solution. After the pH of the lower layer solution was adjusted to 8-11, the solution was layered, and the upper layer was a yellow transparent liquid. The yellow transparent liquid in the upper layer was vacuum-dried at 70° C. to obtain 11.8 g of the product, with a yield of 30.3%. After silica gel column chromatography, a colorless and transparent liquid product with a purity of 99.85% was obtained, and its main physical parameters and HR-MS, FT-IR, ESI + -MS, 1 H-NMR and 13 C-NMR characterization data are as follows:
[0051] Boiling point B.p.=229.3°C; Refractive index ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com