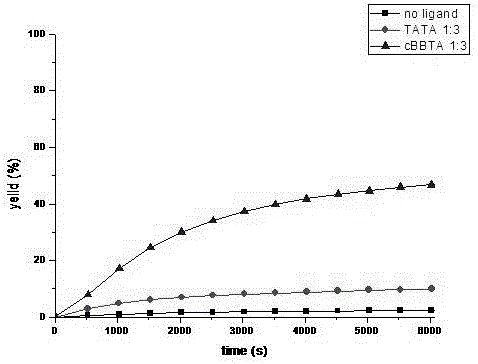
Preparation method of 1,2,3-bis-triazole ligands and application of 1,2,3-bis-triazole ligands in CuAAC reaction
A technology of bistriazole and ligand is applied in the field of synthesis of functional structural molecules, which can solve the problems of inconvenient mass synthesis, difficulty in mass synthesis, harsh reaction conditions, etc. low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
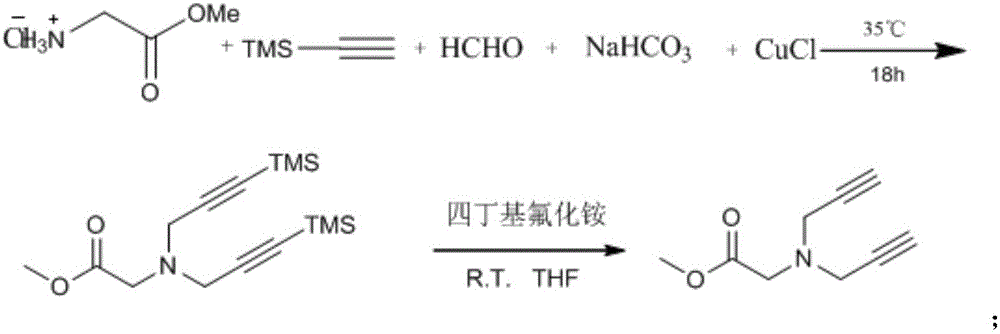
[0029] To synthesize glycine methyl ester TMS-diyne, add glycine methyl ester hydrochloride (1.251g, 10mmol, 1equiv.), trimethylsilyl acetylene (3.27mL, 25mmol, 2.5equiv.), mass 37% aqueous formaldehyde solution (1.85mL, 25mmol, 2.5quiv.), sodium bicarbonate (0.84g, 25mmol, 1equiv.) and cuprous chloride (0.1g, 1mmol, 1equiv.), mixed and stirred at 35°C After 18 hours, the progress of the reaction was monitored by TLC. After the reaction was completed, it was extracted three times with dichloromethane / water, the organic phase was washed and dried, and separated by silica gel column chromatography to obtain 2.86 g of pure target product with a yield of 92%. The product is a colorless liquid. 1 H NMR (CDCl 3, 300 MHz, 22 °C): 3.72 (s, 3H),3.53 (s, 4H), 3.43 (s, 2H), 0.15 (s, 18H). 13 C NMR (CDCl 3 , 75 MHz, 22 °C):170.76, 100.41, 90.72, 53.25, 51.86, 43.88, -0.08.HRMS (ESI) calculated forC 15 h 28 NO 2 Si 2 ([M+H] + ) 310.1653, found 310.1647.
Embodiment 2
[0031] To synthesize glycine methyl ester diyne, add glycine methyl ester TMS-diyne (3.11g, 10mmol, 1equiv.) in 10mL tetrahydrofuran solution in a 100mL round bottom flask, and then add tetrabutylammonium fluoride (7.9g, 25mmol , 2.5equiv,), mixed and stirred at room temperature for 3 hours, the reaction process was monitored by TLC. After the reaction was completed, it was extracted three times with dichloromethane / water, and the organic phase was washed and dried, and separated by silica gel column chromatography to obtain 1.49 g of pure target product with a yield of 90%. The product is a colorless liquid. 1 H NMR (CDCl 3 , 300 MHz, 22 °C): 1 H NMR (400 MHz, CDCl 3 ) δ 3.73 (s,3H), 3.56 (s, 4H), 3.47 (s, 2H), 2.28 (s, 2H). 13 C NMR (CDCl 3 , 75 MHz, 22 °C):170.76, 100.41, 90.72, 53.25, 51.86, 43.88, -0.08.HRMS (ESI) calculated forC 9 h 11 NO 2 ([M+H] + ) 165.0790, found 165.0812.
Embodiment 3
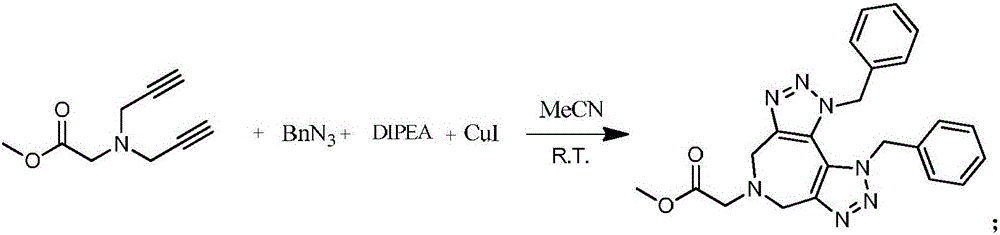
[0033] Add 1mmol glycine methyl ester diyne, 2mmol benzyl azide, 1.2mmol N,N-diisopropylethylamine and 0.1mmol cuprous iodide into a 10mL round bottom flask, mix and stir at room temperature for 3 hours, and react The process was monitored by TLC. After the reaction was completed, it was extracted with ethyl acetate, and the organic phase was washed and separated by silica gel column chromatography to obtain the pure product of the target product. The yield was 85%. The product is a viscous liquid. 1 H NMR (400 MHz, CDCl 3 ) δ 7.38-7.28 (m, 6H), 6.85-6.79 (m, 4H),5.57 (s, 4H), 4.25 (s, 4H), 3.75 (s, 3H), 3.38 (s, 2H). 13 C NMR (101 MHz, CDCl 3 ) δ 170.8, 146.9, 134.0, 129.1, 128.6, 126.1, 123.2, 56.5, 53.3, 52.0, 50.6. HRMS (ESI) m / z calculate for (M+H + ) C 23 h 24 N 7 o 2 + 430.1986, Found: 430.1977.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com