Heterocyclic modulators of lipid synthesis for use against cancer and viral infections
A technology of heterocycles, compounds, in the field of lipid synthesis heterocycle regulators for use against cancer and viral infections
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
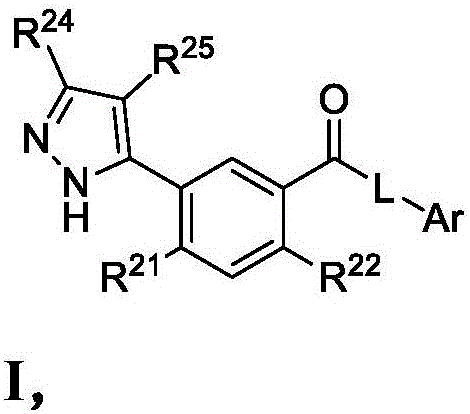
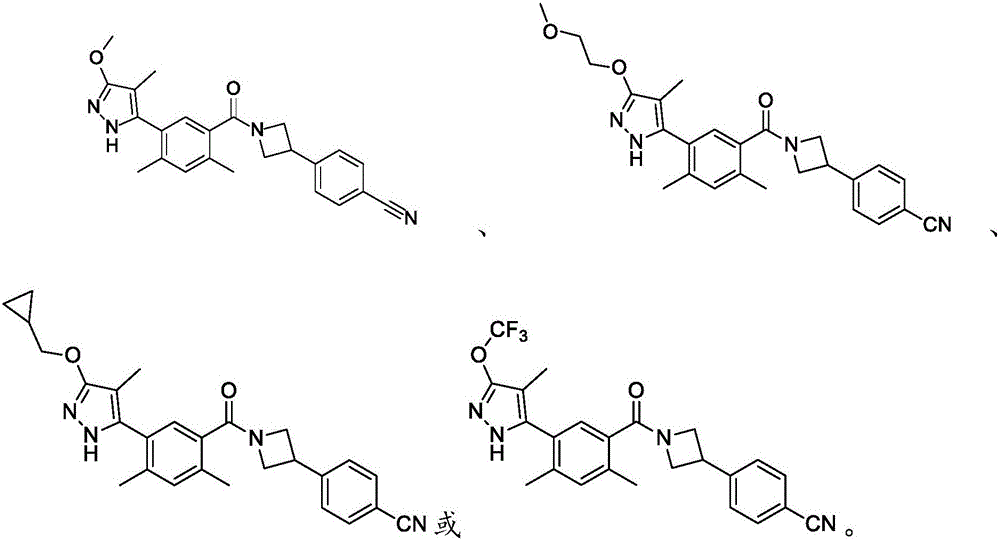
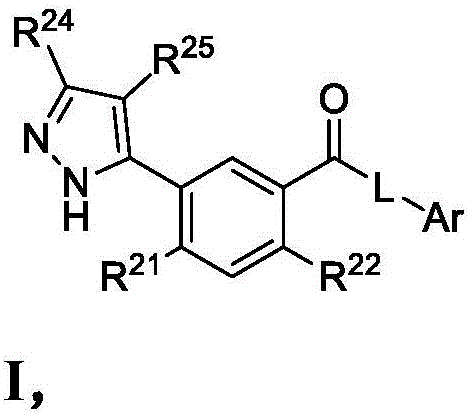
[0164] Synthesis of Compounds of the Disclosure
[0165] General: All reactions and manipulations described were performed in a well-ventilated fume hood. Operations and reactions under increased or reduced pressure are carried out behind explosion-proof barriers. Abbreviations: ACN, acetonitrile; AcOH, acetic acid; AIBN, azobisisobutyronitrile; BF 3 -Et 2 O, boron trifluoride diethyl etherate complex; (Boc) 2 O, di-tert-butyl dicarbonate; BuLi, butyllithium; CDI, 1,1′-carbonyldiimidazole; DBU, 1,8-diazabicyclo[5.4.0]undec-7-ene; DCE , 1,2-dichloroethane; DCM, dichloromethane or methylene chloride; DIEA, N,N-diisopropylethylamine; DMA, N,N-dimethylethylamine Amide; DMAP, 4-dimethylaminopyridine; DME, 1,2-dimethoxyethane; DMEDA-N,N'-dimethylethylenediamine; DMF, N,N-dimethylformamide ; DMSO, dimethylsulfoxide; DPPP, 1,3-bis(diphenylphosphino)propane; EDC, 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide; EDCI , 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride; ...
Embodiment 2
[0245] Activity of Compounds of the Disclosure
[0246] Inhibition of FASN by Compounds of the Disclosure
[0247] Determination of FASN biochemical activity: FASN enzyme was isolated from SKBr3 cells. SKBr3 is a human breast cancer cell line with high FASN expression levels. It is estimated that FASN accounts for approximately 25% of the cytosolic proteins in this cell line. SKBr3 cells were homogenized in a dounce homogenizer and then centrifuged at 4°C for 15 minutes to remove particulate matter. Supernatants were then analyzed for protein content, diluted to appropriate concentrations, and used to measure FASN activity. The presence of FASN was confirmed by Western blot analysis. A similar method for isolating FASN from SKBr3 cells is described in Teresa, P. et al. (Clin. Cancer Res. 2009; 15(24), 7608-7615).
[0248] FASN activity of extracts from SKBr3 cells was determined by measuring the amount of thiol-containing coenzyme A (CoA) oxidized or released by NADPH...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com