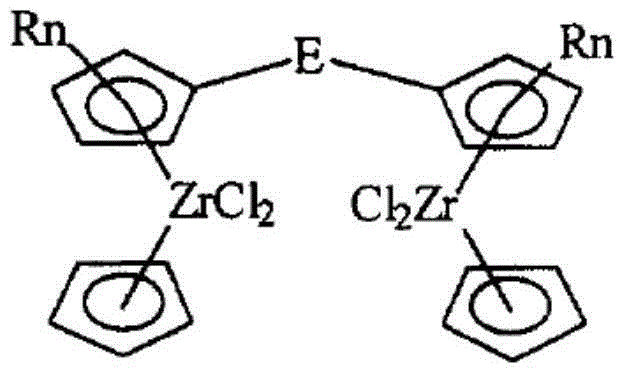
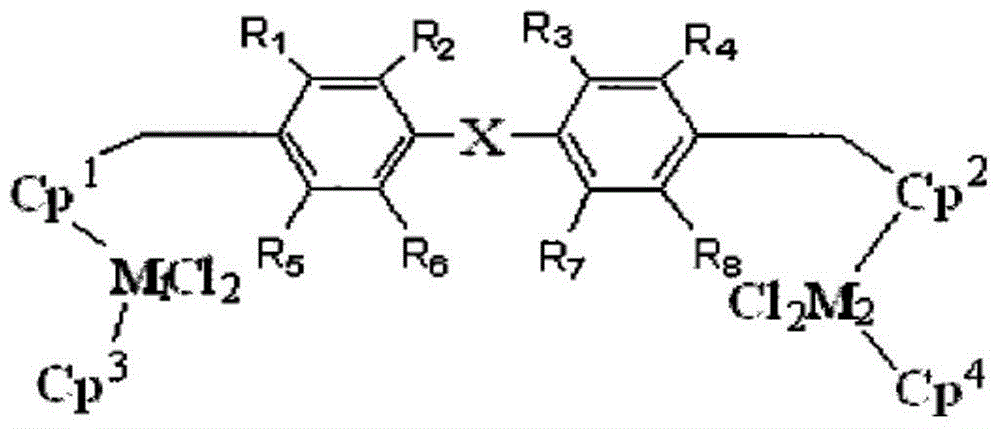
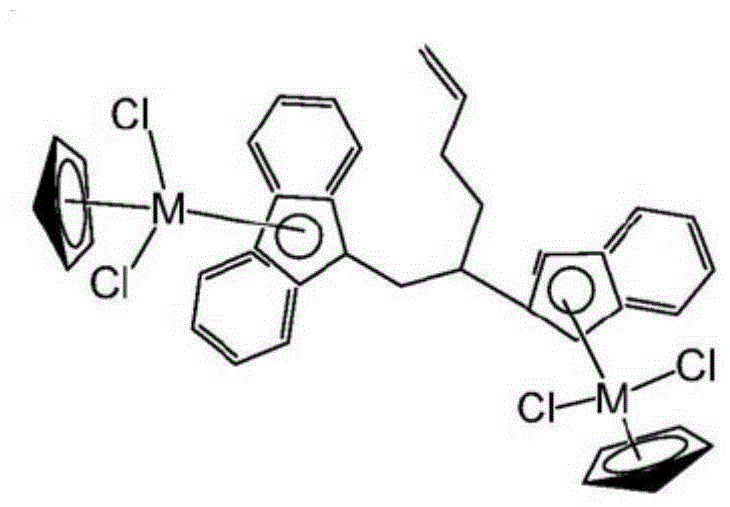
Dinuclear metallocene compound, and preparation method and application thereof
A technology of metallocene compounds and compounds, applied in metallocenes, chemical instruments and methods, organic chemistry, etc., can solve the problems of fast chain transfer, small steric hindrance of substituent groups, and insufficient rigidity, and achieve good mechanical properties and processing performance, wide application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] (1) Synthesis of binuclear metallocene compound A
[0041] Add 0.25mol 4,4-dibromodiphenyl ether and 100mL dry diethyl ether to a 500mL three-necked flask, then add 3.5g lithium metal at -30°C and react for 12 hours, then add 0.5mol monomethyltrichlorosilane After reacting in an ice-water bath for 6 hours, add 100 mL of distilled water to wash, remove the organic phase with a pear-shaped separatory funnel, wash the water phase with 50 mL of ether three times, combine the organic phases, and wash with anhydrous MgSO 4 Dry, filter, remove the solvent in vacuo, and then recrystallize from ethanol to obtain 33.84 g of colorless crystals, with a yield of 48%.
[0042] Dissolve the above compound in 100 mL of dry tetrahydrofuran, slowly add 0.48 mol cyclopentadiene sodium dropwise at -30°C for 12 hours, remove the solvent in vacuo, and extract the residual solid with 50 mL of ether three times, then place it in an ice-water bath Slowly add 192mL of 2.5M n-butyllithium hexane...
Embodiment 2
[0048] (1) Synthesis of binuclear metallocene compound B
[0049] Add 0.12mol 4,4-dibromodiphenyl ether and 80mL dry ether to a 500mL three-necked flask, then add 1.7g lithium metal at -30°C and react for 12 hours, then add 0.25mol monomethyltrichlorosilane After reacting in an ice-water bath for 6 hours, add 80 mL of distilled water to wash, remove the organic phase with a pear-shaped separatory funnel, wash the water phase with 30 mL of ether three times, combine the organic phases, and wash with anhydrous MgSO 4 Dry, filter, remove the solvent in vacuo, and then recrystallize from ethanol to obtain 16.6 g of colorless crystals, with a yield of 48%.
[0050] Dissolve the above compound in 100 mL of dry tetrahydrofuran, slowly add 0.24 mol of indenyllithium dropwise at -30°C for 12 hours, remove the solvent in vacuo, extract the residual solid with 50 mL of ether three times, and then slowly drop it in an ice-water bath Add 81mL of 2.5M n-butyllithium hexane solution, then s...
Embodiment 3
[0056] (1) Synthesis of binuclear metallocene compound C
[0057] Add 0.06mol 4,4-dibromodiphenyl ether and 60mL dry diethyl ether to a 500mL three-necked flask, then add 0.84g lithium metal at -30°C and react for 12 hours, then add 0.12mol phenyltrichlorosilane After reacting in an ice-water bath for 6 hours, add 60 mL of distilled water to wash, remove the organic phase with a pear-shaped separatory funnel, wash the water phase with 30 mL of ether three times, combine the organic phases, and wash with anhydrous MgSO 4 Dry, filter, remove the solvent in vacuo, and then recrystallize from ethanol to obtain 14.04 g of colorless crystals, yield 45%.
[0058] Dissolve the above compound in 100 mL of dry tetrahydrofuran, slowly add 0.12 mol indenyllithium dropwise at -30°C for 12 hours, remove the solvent in vacuo, extract the residual solid with 50 ethyl ether three times, and then slowly drop it in an ice-water bath Add 40mL of 2.5M n-butyllithium hexane solution, then stir at ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com