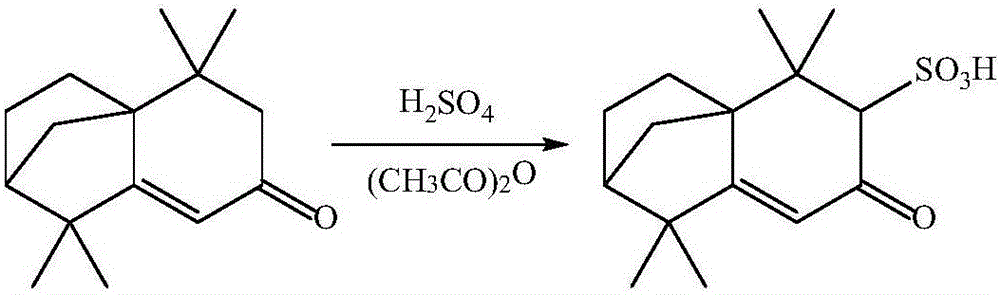
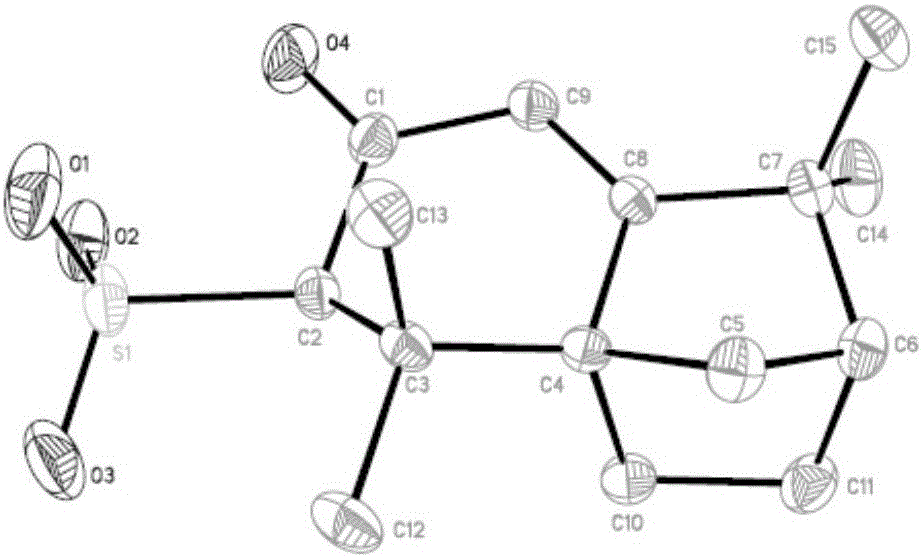
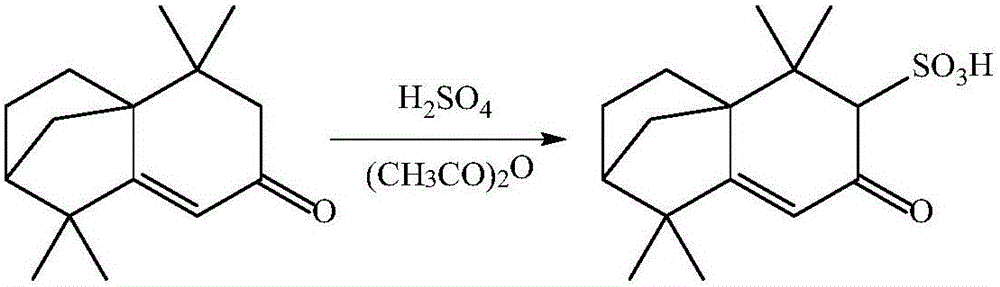
Preparation method of isolongifolenonesulfonic acid
A technology of phyllene sulfonic acid and isolongifolene sulfonic acid, which is applied in the field of synthesis of isolongifolene sulfonic acid, can solve the problems of synthesizing camphor without optical activity, long production cycle, complicated process, etc., and achieve broadening of deep processing and The effect of comprehensive utilization of channels, low price, and simple process operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] In a 100ml three-necked flask, add 18ml of the acetic anhydride (0.15mol), then add 13.09g (0.06mol) of isolongifolenone, and stir with a magnetic stirrer for 1h under ice-bath conditions, and then pass constant pressure drop Add the concentrated sulfuric acid of 5ml (0.078mol) in the three-necked flask by funnel, then react 2h under ice-bath condition, remove ice-bath, react 8h at room temperature, add 10ml ethyl acetate in the emulsion after reaction, then Place the emulsion in a 100ml beaker and let it stand for 24h, filter the emulsion with suction to obtain a white solid filter cake, wash the white solid filter cake with ethyl acetate, place the white solid filter cake in a vacuum drying oven at 40~ Dry at 80°C for 5 hours to obtain isolongifolene sulfonic acid, and the chemical formula for synthesizing isolongifolene sulfonic acid is as follows figure 1 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com