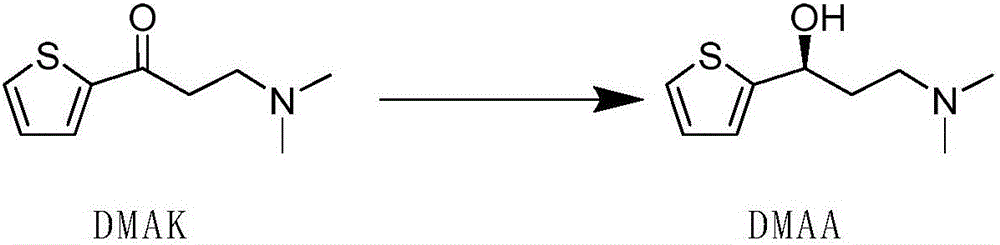
Engineered ketoreductase polypeptide and method for preparing (S)-3-(dimethylamino)-1-(thiophene -2-yl)-1-propanol by using same
A dimethylamine-based, reductase technology, applied in the directions of oxidoreductase, microorganism-based methods, biochemical equipment and methods, etc., can solve the problems of low substrate concentration, high enzyme amount, long reaction time, etc. The effect of high concentration, low enzyme dosage and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Embodiment 1 (preparation of ketoreductase)
[0016] A ketoreductase catalyst was prepared by a conventional method: 1, 3, 5, 7, 9, 11, 13, 15, 17, 19, 21, 23, 25, 27, 29, 31, 33, 35, The gene fragments of 37, 39, 41, and 43 were synthesized by Suzhou Jinweizhi Biotechnology Co., Ltd., ligated with the digested product of pET30a (Novagen) plasmid, transferred into competent E.coli BL21 (DE3) strain, and screened to obtain positive clones , inoculated into liquid LB medium containing resistance, cultivated at 37°C until OD600 to 0.8, added the inducer IPTG, continued to cultivate for 16 hours, collected the precipitate by centrifugation, added phosphate buffer to suspend, and ultrasonicated for 10 minutes in an ice-water bath , centrifuge to get the supernatant, and freeze to obtain ketoreductase enzyme powder.
Embodiment 2
[0017] Embodiment 2 (screening of ketoreductase)
[0018] Expressed in E. coli by the sequences 1, 3, 5, 7, 9, 11, 13, 15, 17, 19, 21, 23, 25, 27, 29, 31, 33, 35, 37, 39, 41, 43 Add 2 mg of the obtained ketoreductase, 2 mg of GDH (purchased from Suzhou Hanzyme Biotechnology Co., Ltd., brand EW002), 1 mg of NADP, 50 mg of substrate DMAK, and 50 mg of glucose into a 5 mL reactor containing 2 mL of 0.05M triethanolamine buffer , stirred at 1000rpm at 30°C, sampled for HPLC detection after 1h, the conversion rate and ee of sequence 43 were both >99%, so sequence 43 was used as the object of further research.
Embodiment 3
[0019] Embodiment 3 (enzyme catalyzed reaction)
[0020] Add 2.0g of substrate DMAK, 3g of glucose, and 9mL of 0.05M pH 7.0 triethanolamine buffer solution to a 50mL three-necked reaction flask in sequence, adjust the temperature to 30°C, stir at 900r / min, and use 15% Na 2 CO 3 The solution maintains the pH of the reaction system to 7.0, and 6 mg of ketoreductase (obtained by expressing sequence 43 in Escherichia coli), 6 mg of glucose dehydrogenase (purchased from Suzhou Hanzyme Biotechnology Co., Ltd., brand EW002) and 0.5mg of NADP in triethanolamine buffer solution, reacted for 12h, the conversion rate was 99% as detected by HPLC, heated and concentrated to a volume of 10mL, filtered to take the filtrate, adjusted the pH to 12.0-12.5 with a 25% NaOH solution, and cooled to 0°C, heat preservation for 12 hours, and filter to obtain 1.7 g of the product with a content of 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com