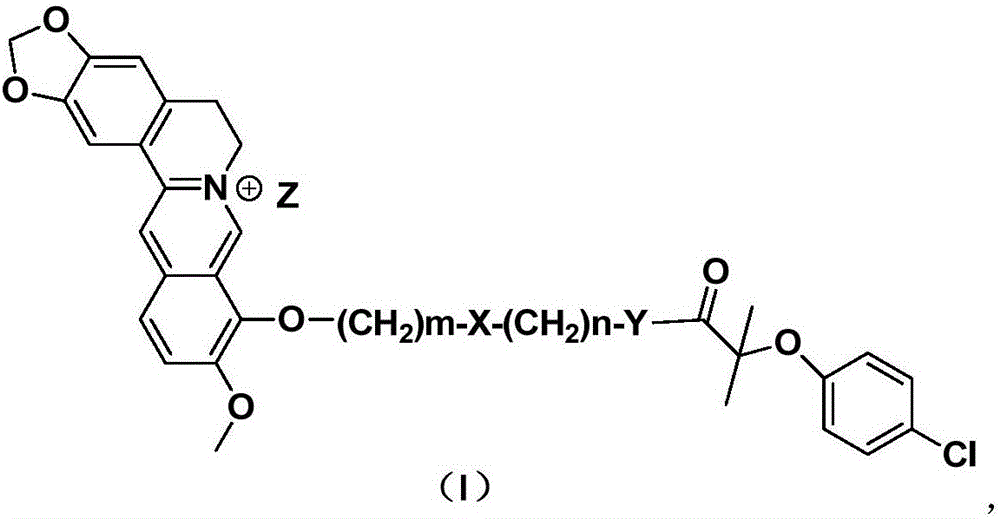Preparation method and application of 9-substituted dual functional group berberine derivative
A derivative, the technology of berberine, applied in the field of food and pharmacy, can solve the problems of low water solubility, low bioavailability and low fat solubility of berberine hydrochloride
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
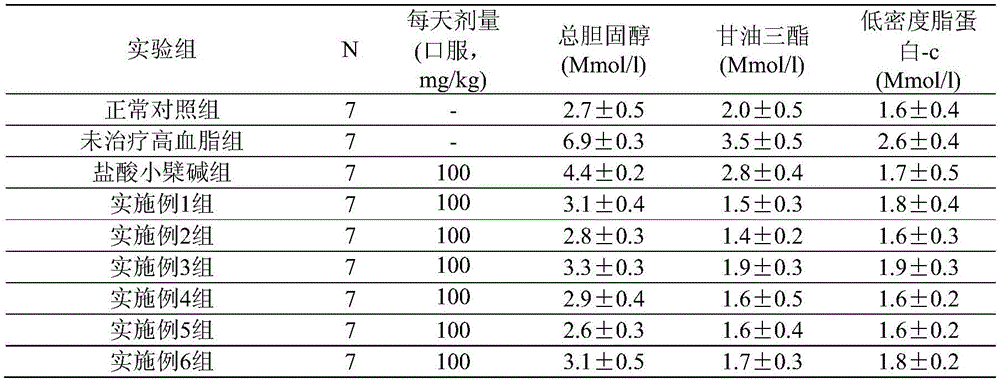
Examples
Embodiment 1
[0013] a) Synthesis of Berberine
[0014] Add 7.4g of berberine to a 250mL round flask, heat for about 30min at a vacuum of 20-30mmHg, 190-200°C, the yellow solid gradually turns dark red, cool to room temperature in a vacuum desiccator, and purify by silica gel column chromatography , to obtain dark red powder 4.7g, yield 75%.
[0015] b) Synthesis of 2-(4-chlorophenoxy)-2-methylpropionic acid-2-bromoethyl ester
[0016] Add 2-(4-chlorophenoxy)-2-methylpropionic acid (4.28g0.02mol) to a 100mL round bottom flask, add 10mL DMF to dissolve, stir at room temperature, add sodium hydroxide (0.96g, 0.024mol), Stir for 10 minutes, then add (7.5g, 0.04mol) 1,2-dibromoethane, raise the temperature to 70°C, stir for about 5 hours, follow the reaction by TLC, add 20ml of water after the reaction is complete, and extract with ethyl acetate 20mL*2 For the second time, the organic phases were combined, dried, filtered, concentrated under reduced pressure, purified by silica gel column chr...
Embodiment 2
[0020] a) Synthesis of Berberine
[0021] Same as a) in Example 1
[0022] b) Synthesis of 2-(4-chlorophenoxy)-2-methylpropionic acid-3-bromopropyl ester
[0023] Add 2-(4-chlorophenoxy)-2-methylpropionic acid (4.28g, 0.02mol) to a 100mL round bottom flask, add 10mL DMF to dissolve, stir at room temperature, add sodium hydroxide (0.96g, 2.4mmol) , stirred for 10 minutes, then added (8g, 0.04mol) 1,3-dibromopropane, heated to 70°C, stirred for about 5 hours, followed by TLC. After the reaction was complete, 20ml of water was added, and the dibromopropane was extracted with 20mL*2 ethyl acetate. Once, the organic phases were combined, dried, filtered, concentrated under reduced pressure, and purified by silica gel column chromatography to obtain 6 g of 2-(4-chlorophenoxy)-2-methylpropionic acid-3-bromopropyl, with a yield of 90% .
[0024] c) Synthesis of 9-(3-(2-(4-chlorophenoxy)-2-methylpropionic acid)propoxy)-O-berberine hydrobromide
[0025] Add berberine (3.2g, 1mol) to...
Embodiment 3
[0027] a) Synthesis of Berberine
[0028] Same as a) in Example 1
[0029] b) Synthesis of 2-(4-chlorophenoxy)-2-methylpropionic acid-4-bromobutyl
[0030] Add 2-(4-chlorophenoxy)-2-methylpropionic acid (4.3g, 0.02mmol) to a 100mL round bottom flask, add 10mL of DMF to dissolve, stir at room temperature, add sodium hydroxide (1g, 0.025mmol), Stir for 10 minutes, then add (8.6g, 0.04mol) 1,4-butylbromopropane, raise the temperature to 70°C, stir for about 5 hours, follow the reaction by TLC, add 20ml of water after the reaction is complete, and extract the disulfide with ethyl acetate 20mL*2 Once, the organic phases were combined, dried, filtered, concentrated under reduced pressure, and purified by silica gel column chromatography to obtain 5.8 g of 2-(4-chlorophenoxy)-2-methylpropionic acid-4-bromobutyl, yield 83 %.
[0031] c) Synthesis of 9-(4-(2-(4-chlorophenoxy)-2-methylpropionic acid)butoxy)-O-berberine hydrobromide
[0032] Add berberine (3.2g, 1mol) to a 50mL round...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com