Clomiphene synthesis using a single solvent
A technology of organic solvents and solutions, which is applied in the preparation of organic compounds, aminohydroxy compounds, carboxylates, etc., and can solve problems such as difficult assembly lines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
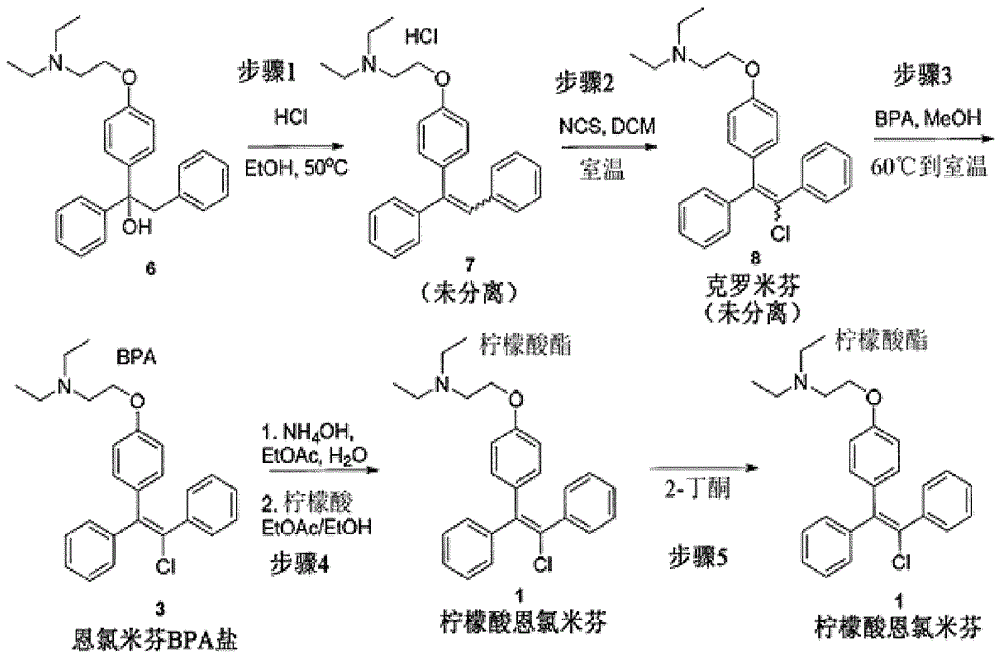
[0025] by 1 -{4-[2-(Diethylamino)ethoxy]phenyl}-1,2-diphenylethanol Preparation of trans-clomiphene citrate
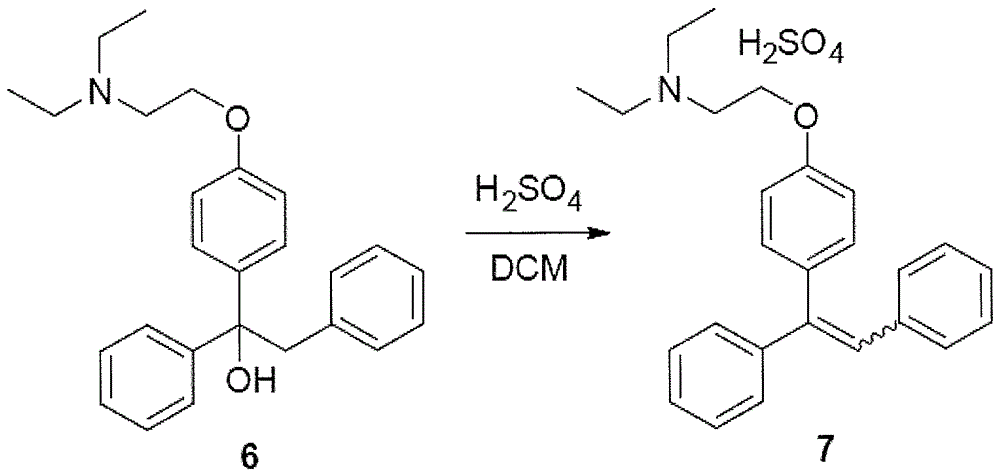
[0026] dehydration
[0027] 1-{4-[2-(Diethylamino)ethoxy]phenyl}-1,2-diphenylethanol (6) dissolved in ethanol containing excess hydrochloric acid was refluxed at 50°C for 3 hours. The solvent and excess hydrochloric acid were removed in vacuo and the residue was dissolved in dichloromethane. 2-{4-[(Z)-1,2-diphenylethenyl]phenoxy}-N,N-diethylethylammonium hydrochloride (7) is obtained.
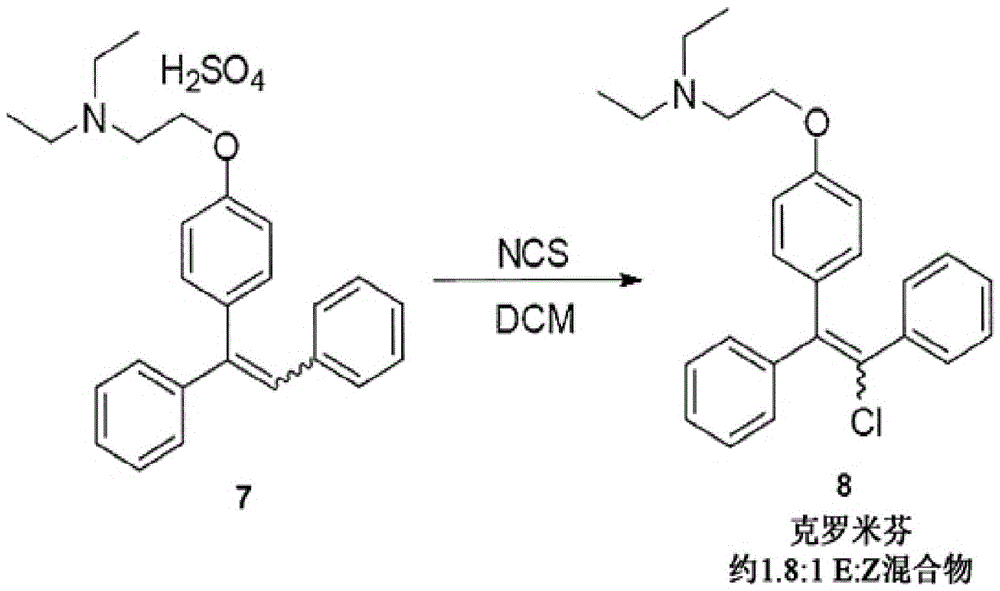
[0028] Chlorination
[0029] The hydrochloride (7) solution obtained above was treated with 1.05 equivalents of N-chlorosuccinimide and stirred at room temperature for about 20 hours. Reaction completion was confirmed by HPLC. The hydrochloride salt was converted to the free base by addition of saturated aqueous bicarbonate solution. The mixture was stirred at room temperature for 30 minutes, then the phases were separated and the organic phase was evaporated in vacuo. 2-{4-...
example 2
[0034] Synthesis of Clomiphene Using a Single Solvent
[0035] Step 1 - Dehydration of 1-{4-[2-(diethylamino)ethoxy]phenyl}-1,2-diphenylethanol to form sulfuric acid Hydrogen 2-{4-[(Z)-1,2-diphenylvinyl]phenoxy}-N,N-diethylethylammonium (7)
[0036] The synthetic route described in Example 1 used HCl for the dehydration step and ethanol at 50°C as solvent. Due in part to the more favorable corrosion properties of sulfuric acid, sulfuric acid was investigated as an alternative to HCl for the dehydration step (as described in Example 1). Dichloromethane (chlorinated methane) was investigated as an alternative solvent for the dehydration step as this would eliminate the need to remove the ethanol solvent prior to the chlorination step.
[0037] Mix 1-{4-[2-(diethylamino)ethoxy]phenyl}-1,2-diphenylethanol (6) (6.60g, 16.9mmol) and 66mL (1×10 3mmol) of methyl chloride was charged to a 100 mL 3-neck round bottom flask equipped with a temperature probe and a stir bar to give ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com