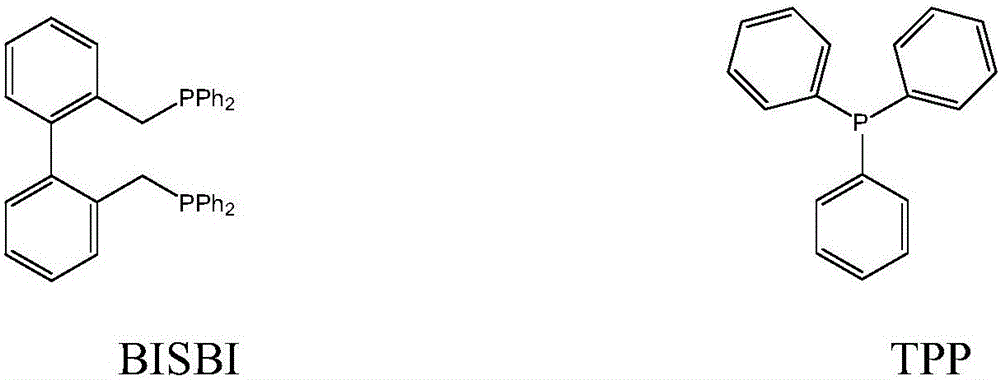Method for preparing butyraldehyde through propylene hydroformylation
A technology for propylene hydroformylation and butyraldehyde is applied in the field of propylene hydroformylation to prepare butyraldehyde, and can solve the problems of complex preparation process, high price, and few applications of BISBI.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
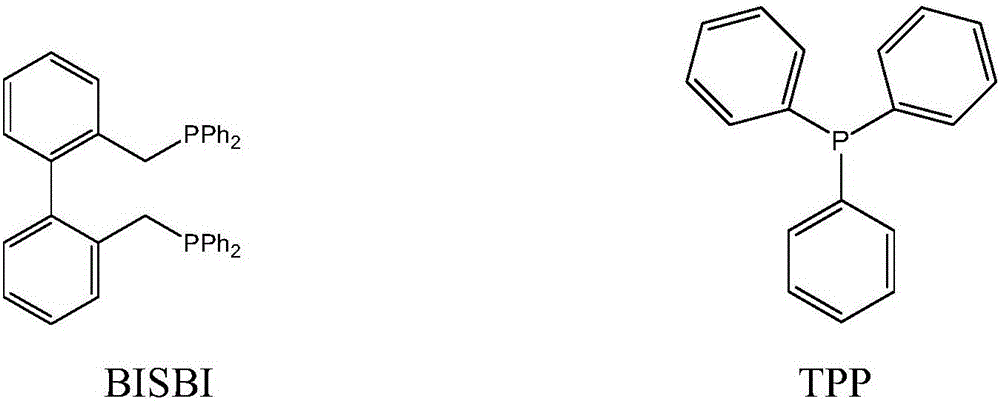
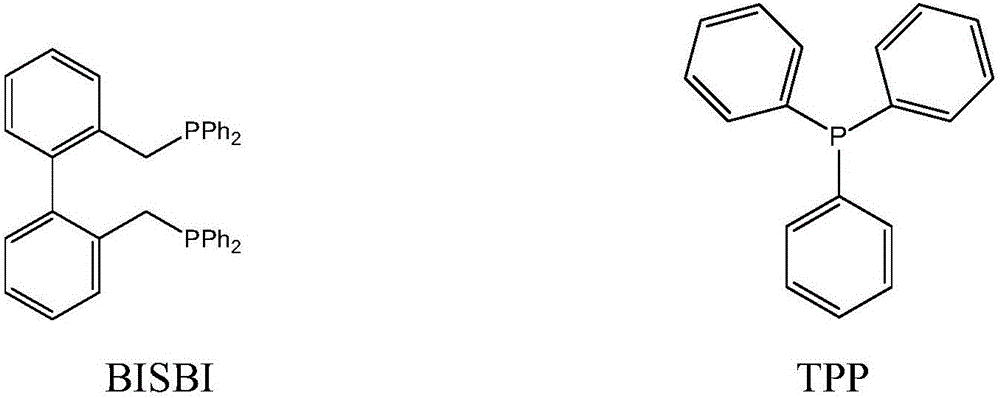
[0045] 100g of toluene, the molar concentration is 1.5×10 -3 Mole / L rhodium catalyst HRh(CO)(TPP) 3 , 6×10 -2 Mol / L bisphosphine ligand BISBI, 3×10 -3 Mole / L of TPP, put it into a 250 ml autoclave with a stirrer, use H in the autoclave 2 : Replace the syngas with CO=1:1 (molar ratio) for 3 to 5 times, then add 27 grams of propylene, and then add syngas to the total pressure in the kettle to 1.6MPa, and react for 1 hour at a reaction temperature of 85°C and a stirring speed of 200rpm After cooling to room temperature and releasing unreacted gas, the reaction solution was analyzed by gas chromatography, and the conversion rate of propylene was 85%, and the selectivity of butyraldehyde produced was 98%, among which n-butyraldehyde and isobutyraldehyde The molar ratio is 25:1.
Embodiment 2
[0047] 125 grams of anisole, the molar concentration is 2×10 -3 Mole / L rhodium catalyst RhCl(CO)(TPP) 2 , 4×10 -2 Mol / l BISBI, 6×10 -3 Mole / L of TPP was added to a 250 ml autoclave with a stirrer, and H was used in the autoclave 2 : CO=1:1 (molar ratio) syngas replacement 3 to 5 times, then add 30 grams of propylene, then add syngas to the total pressure in the kettle to 1.8MPa, react at a reaction temperature of 90°C and a stirring speed of 200rpm for 1 hour, After the reaction liquid in the autoclave was cooled to room temperature, unreacted gas was released. The reaction liquid was taken out and analyzed by gas chromatography. The conversion rate of propylene was determined to be 94%, and the selectivity to butyraldehyde was 97%. The molar ratio with isobutyraldehyde is 21:1.
Embodiment 3
[0049] Put 100 grams of n-butyraldehyde with a molar concentration of 2.5×10 -3 Mol / L rhodium catalyst Rh(CO) 2 (acac), 8×10 -3 Mole / L BISBI, 5×10 -2 Mole / L of TPP was added to a 250 ml autoclave with a stirrer, and H was used in the autoclave 2 : CO=1:1 (molar ratio) syngas replacement 3 to 5 times, then add 40 grams of propylene, then add syngas to the total pressure in the kettle to 2.0MPa, react at a reaction temperature of 100°C and a stirring speed of 200rpm for 1 hour, After the reaction liquid in the autoclave was cooled to room temperature, unreacted gas was released. The reaction liquid was taken out and analyzed by gas chromatography. The conversion rate of propylene was calculated to be 96%, and the selectivity to butyraldehyde was 97%. The ratio of butyraldehyde is 15:1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com