A kind of 2,5-disubstituted-1,3,4-oxadiazole derivative and its application
A technology of oxadiazole and derivatives, applied in the field of pesticides, can solve problems such as the reduction of the sensitivity of pest populations, and achieve the effect of alleviating the problem of drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
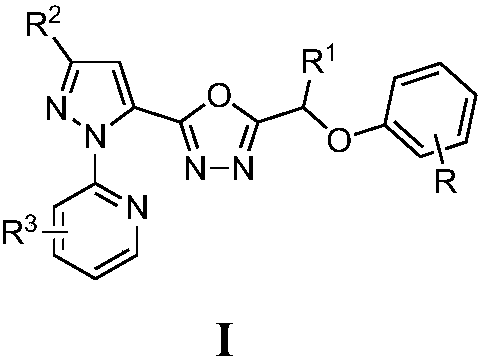
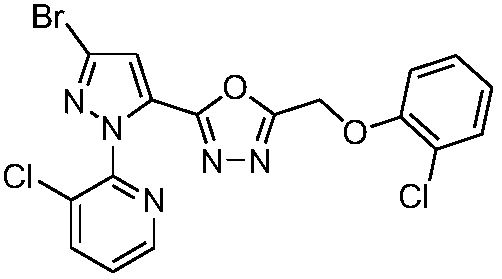
[0022] Compound I-1:: 2-[5-(3-bromo-1-(3-chloro-pyridin-2-yl)-1H-pyrazole)]-5-(2-chlorophenoxymethylene)- 1,3,4-oxadiazole
[0023]
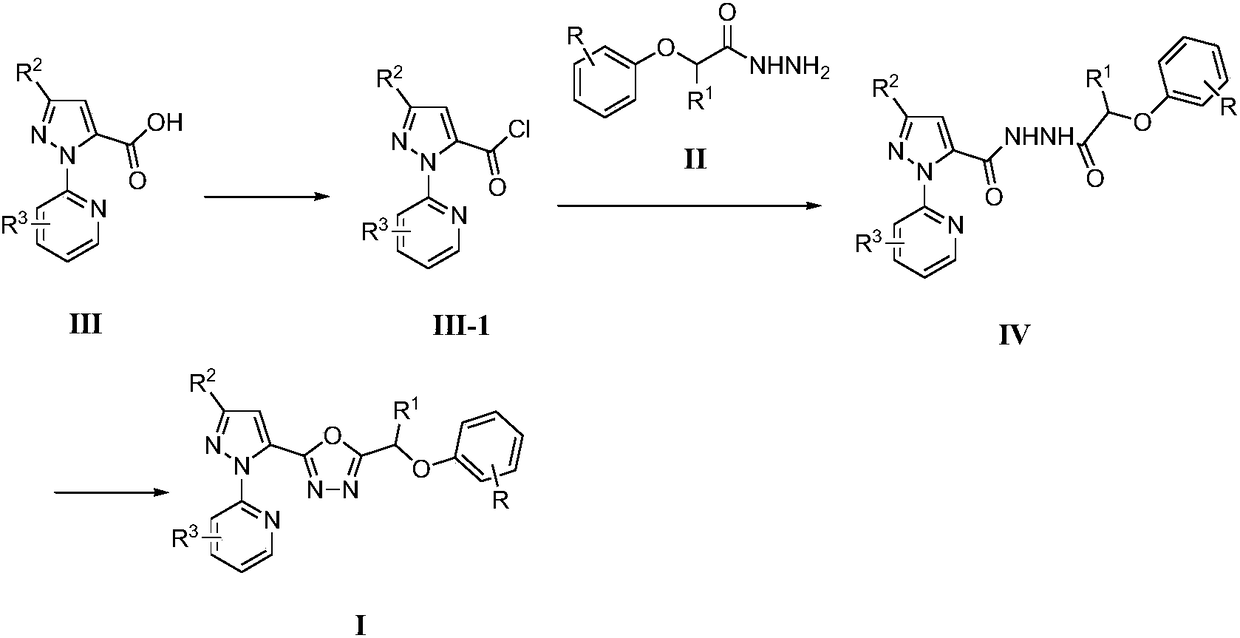
[0024] (1) Take 5.5mmol of 3-bromo-1-(3-chloropyridin-2-yl)-1H-pyrazole-5-carboxylic acid III, 3mL of thionyl chloride and 7mL of toluene and add them into a reflux condenser and a thermometer In a 25mL three-necked flask, heat up to 50-80°C for 4-5 hours, then distill off excess thionyl chloride and toluene to obtain 3-bromo-1-(3-chloropyridin-2-yl)- 1H-pyrazole-5-formyl chloride III-1, directly used in the next step reaction;
[0025] (2) In a 50mL round bottom flask, add 5mmol intermediate 4-chlorophenoxyalkyl hydrazide II, 3mmol K 2 CO 3 , 20mL of dichloromethane, the intermediate 3-bromo-1-(3-chloropyridin-2-yl)-1H-pyrazole-5-carbonyl chloride III-1 was added dropwise under stirring at room temperature, after the addition was completed, the reaction was tracked by TLC. Stir at room temperature for 0.5-3h, filter with suction, wash wi...
Embodiment 2
[0069] Compound I-7: 2-[5-(3-bromo-1-(3-chloro-pyridin-2-yl)-1H-pyrazole)]-5-(2,4-dichlorophenoxymethylene )-1,3,4-oxadiazole
[0070]
[0071] (1) Take 5.5mmol of 3-bromo-1-(3-chloropyridin-2-yl)-1H-pyrazole-5-carboxylic acid III, 3mL of thionyl chloride and 7mL of toluene and add them into a reflux condenser and a thermometer In a 25mL three-necked flask, heat up to 50-80°C for 4-5 hours, then distill off the remaining thionyl chloride and toluene to obtain 3-bromo-1-(3-chloropyridin-2-yl)- 1H-pyrazole-5-formyl chloride III-1, directly used in the next step reaction;
[0072] (2) Add 5mmol intermediate 2,4-dichlorophenoxyalkylhydrazide II, 3mmol K to a 50mL round bottom flask 2 CO 3 , 20mL of dichloromethane, the intermediate 3-bromo-1-(3-chloropyridin-2-yl)-1H-pyrazole-5-carbonyl chloride III-1 was added dropwise under stirring at room temperature, after the addition was completed, the reaction was tracked by TLC. Stir at room temperature for 0.5-3h, filter with suct...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com