Purine alanine derivative used for tumor therapy, and preparation method and application of derivative
A technology for tumor treatment and alanine, which is applied in the production of antineoplastic drugs, drug combinations, and bulk chemicals. Novelty effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
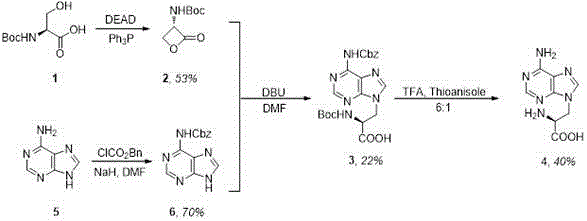
[0031] The specific reaction steps of synthetic 9-adenine-L-alanine include:
[0032] Step 1) Synthesis of N-(tert-butoxycarbonyl 1)-L-serine-lactone 2: In triphenylphosphine (Ph 3 Add anhydrous tetrahydrofuran (THF) (100mL) dropwise to P) (6.3g, 24.00mmol), stir for more than 10min at -78°C under argon atmosphere, then add diethyl azodicarboxylate (DEAD) dropwise , and the resulting solution was stirred for 10 min. The above tetrahydrofuran mixed solution was added dropwise to N-(tert-butoxycarbonyl 1)-L-serine (5 g, 24.36 mmol), and stirred for more than 20 min. The obtained solution was stirred at -78° C. for 20 min, and then further stirred at room temperature for 2.5 h. The solvent tetrahydrofuran was removed and the crude product was purified by column chromatography (ethane / ethyl acetate in a volume ratio of 3:1). Finally, recrystallization in ethyl acetate / n-hexane was further purified to obtain white solid compound 2 (2.4 g, yield 53%).
[0033] Step 2) Synthesis ...
Embodiment 2
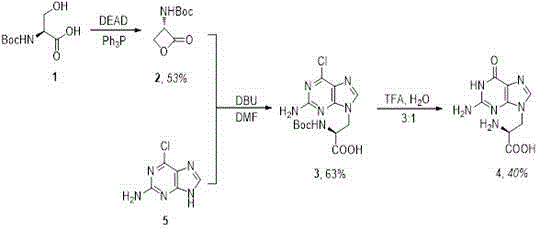
[0039] The specific reaction steps of synthetic 9-guanine-L-alanine include:
[0040] Step 1) Synthesis of N-(tert-butoxycarbonyl 1)-L-serine-lactone 2: In triphenylphosphine (Ph 3 Add anhydrous tetrahydrofuran (THF) (100mL) dropwise to P) (6.3g, 24.00mmol), stir for more than 10min at -78°C under argon atmosphere, then add diethyl azodicarboxylate (DEAD) dropwise , The resulting solution was stirred for 10 min. The above tetrahydrofuran mixed solution was added dropwise to N-(tert-butoxycarbonyl 1)-L-serine (5 g, 24.36 mmol), and stirred for more than 20 min. The resulting solution was stirred at -78°C for 20 min. Then it was further stirred for 2.5 h at room temperature. The solvent was removed and the crude product was purified by column chromatography (ethane / ethyl acetate in a volume ratio of 3:1). Finally, further purification was carried out by recrystallization in ethyl acetate / n-hexane to obtain white solid compound 2 (2.4 g, yield 53%).
[0041] Step 2) Synthesi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com