Bisphosphonate compounds as well as preparation method and application thereof
Technology of a compound, bisphosphonic acid, applied in the field of biomedicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
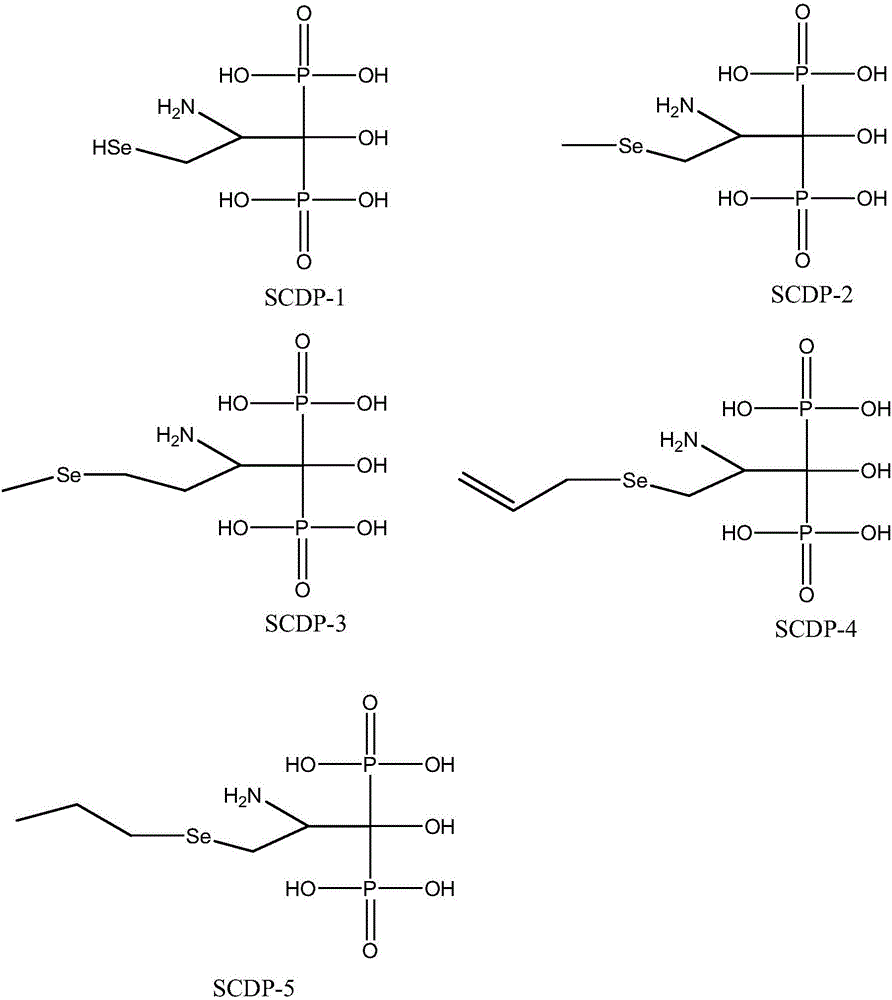
[0055] Embodiment 1: Synthetic SCDP-1 compound
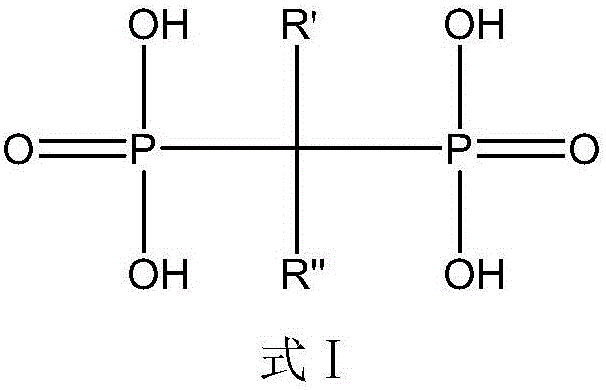
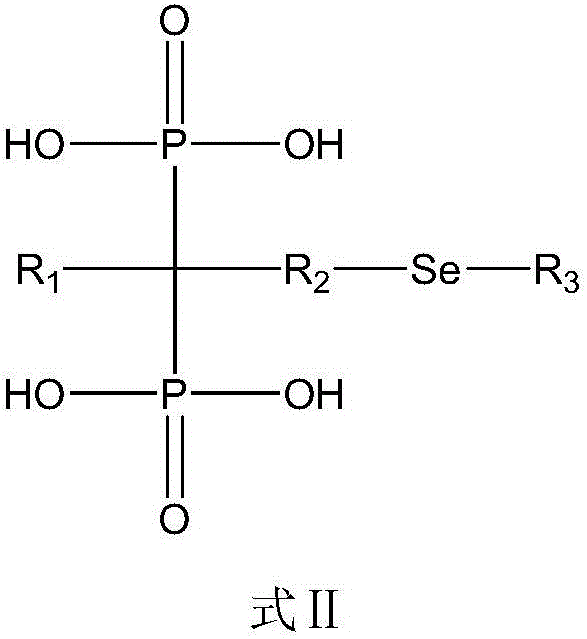
[0056] In this example, the diphosphonic acid compound is: 3-selenohydroxy-2-amino-1-hydroxypropylene-1,1-diphosphonic acid. The structural formula is as follows
[0057]
[0058] The specific preparation method of this compound is as follows:
[0059] Add 1.68 g of 3-selenohydroxy 2-amino-propionic acid into a 50 mL three-necked flask. Add 10 mL of tetrahydrofuran, stir and add 2.4 g of catecholborane, react for 2 hours, then add 9.0 g of tris-(trimethylsilyl) phosphite, react for 2 hours, add methanol 40 mL and react for 2 hours. Distill under reduced pressure until an oily substance was produced, then add 300 mL of diethyl ether to precipitate a precipitate, and recrystallize the precipitate with water to obtain a white solid 3-selenohydroxy-2-amino-1-hydroxypropylidene-1,1-diphosphonic acid.
[0060] The white solid was identified by:
[0061] 1 H-NMR (400MHz, D 2 O) δ 2.65 (T, 1H), δ 1.41-1.72 (D, 2H). MS (ESI, m / ...
Embodiment 2
[0063] Embodiment 2: Synthetic SCDP-2 compound
[0064] In this example, the diphosphonic acid compound is: 3-methylselenyl-2 amino-1 hydroxypropylene-1,1-diphosphonic acid. The structural formula is as follows
[0065]
[0066] The specific preparation method of this compound is as follows:
[0067] Add 1.82 g of 3-methylselenyl-2-amino-propionic acid into a 50 mL three-necked flask. Add 10 mL of tetrahydrofuran, stir and add 2.4 g of catecholborane, react for 2 hours, then add 9.0 g of tris-(trimethylsilyl) phosphite, react for 2 hours, add methanol 40 mL and react for 2 hours. Distill under reduced pressure until an oily substance was produced, then add 300 mL of diethyl ether to precipitate a precipitate, and recrystallize the precipitate with water to obtain a white solid 3-methylseleno-2amino-1-hydroxypropylidene-1,1-diphosphonic acid.
[0068] The white solid was identified by:
[0069] 1 H-NMR (400MHz, D 2 O) δ 2.68 (T, 1H), δ 1.43-1.74 (D, 2H), δ, 0.98 (S, 3H...
Embodiment 3
[0071] Embodiment 3: Synthetic SCDP-3 compound
[0072] In this example, the diphosphonic acid compound is: 4-methylselenyl-2-amino-1 hydroxypropylene-1,1-diphosphonic acid. The structural formula is as follows:
[0073]
[0074] The specific preparation method of this compound is as follows:
[0075] Add 1.96 g of 4-methylselenyl-2-amino-butyric acid into a 50 mL three-necked flask. Add 10 mL of tetrahydrofuran, stir and add 2.4 g of catecholborane, react for 2 hours, then add 9.0 g of tris-(trimethylsilyl) phosphite, react for 2 hours, add methanol 40 mL and react for 2 hours. Distill under reduced pressure until an oily substance was produced, then add 300 mL of diethyl ether to precipitate a precipitate, and recrystallize the precipitate with water to obtain a white solid 4-methylselenyl-amino-1-hydroxybutylene-1,1-diphosphonic acid.
[0076] The white solid was identified by:
[0077] 1 H-NMR (400MHz, D 2 O) δ 2.68 (T, 1H), δ 1.53 (Q, 2H), δ 1.32 (T, 2H) δ, 0.99 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com