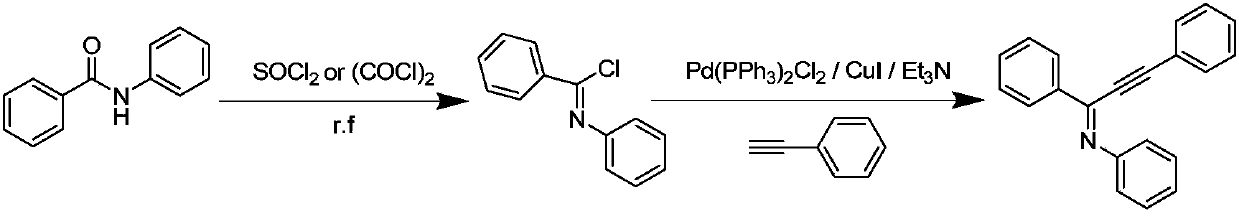
A kind of preparation method of the alkynyl imine compound containing α-h
A technology of alkynyl imines and compounds, which is applied in the field of organic synthesis, can solve the problems of inability to synthesize alkynyl imine compounds, waste of thionyl chloride or oxalyl chloride, cumbersome steps, etc., and achieve strong substrate design and easy Good effect of operation and activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~8
[0038] The molar ratio of raw materials is as follows: phosphoroimide compound (II): acyl chloride (III) containing α-H: alkynyl copper compound (IV)=1:1.2:1.8. Add phosphoroimide (II) into a 50ml Schlenk tube, vacuumize and change nitrogen three times, cool down to -78°C, add α-H-containing acid chloride (III) and 5ml of organic solvent dropwise, stir for 1 hour, then drop into Copper(IV) alkynes prepared in situ. After the dropwise addition was complete, the reaction was moved to room temperature for another 2-3 hours. After the spot plate detection reaction is completed, the reaction is quenched, the liquid is extracted and separated, the sample is mixed with silica gel, and purified by column chromatography (eluent is petroleum ether: ethyl acetate = 30:1) to obtain the corresponding alkynyl group containing α-H Imine compound (I), the yield of each product is shown in Table 1. The reaction process is shown in the following formula:
[0039]
[0040] The reaction yie...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap