Preparation method of (Zn<1-x>Mx)3V2O8
A 3V2O8, zn1-xmx technology, applied in the field of luminescent material preparation, can solve the problems of complex price change, difficult phosphor powder, affecting luminous performance, etc., to achieve the effect of improving performance, covering a wide range and luminous intensity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
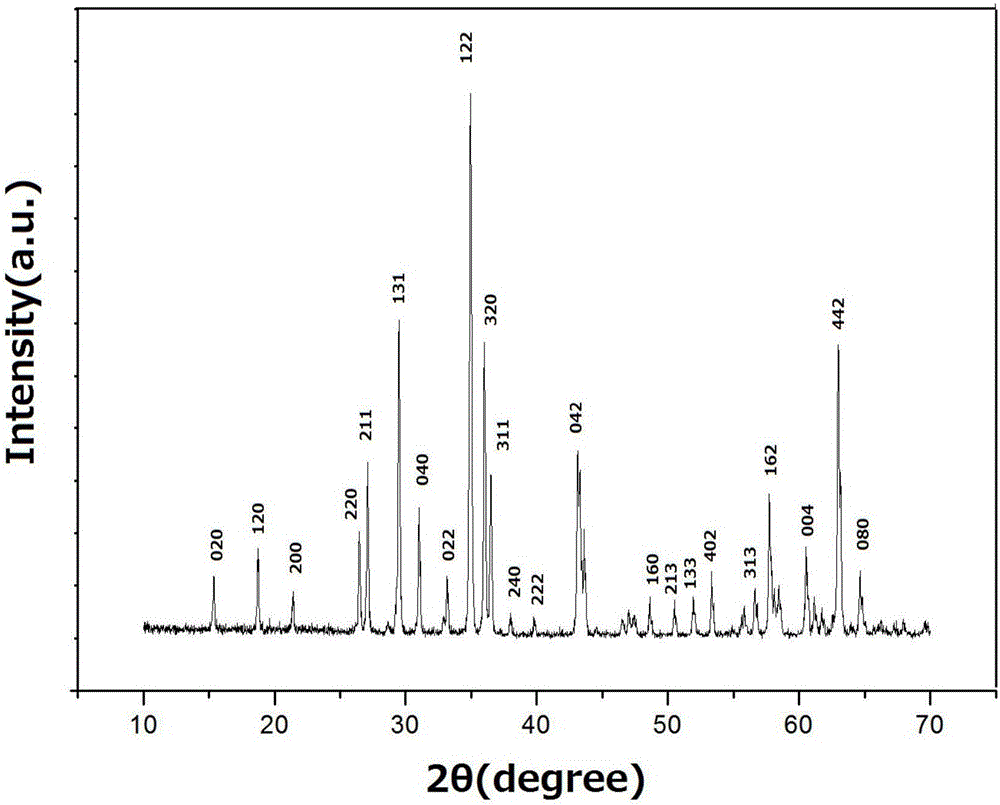
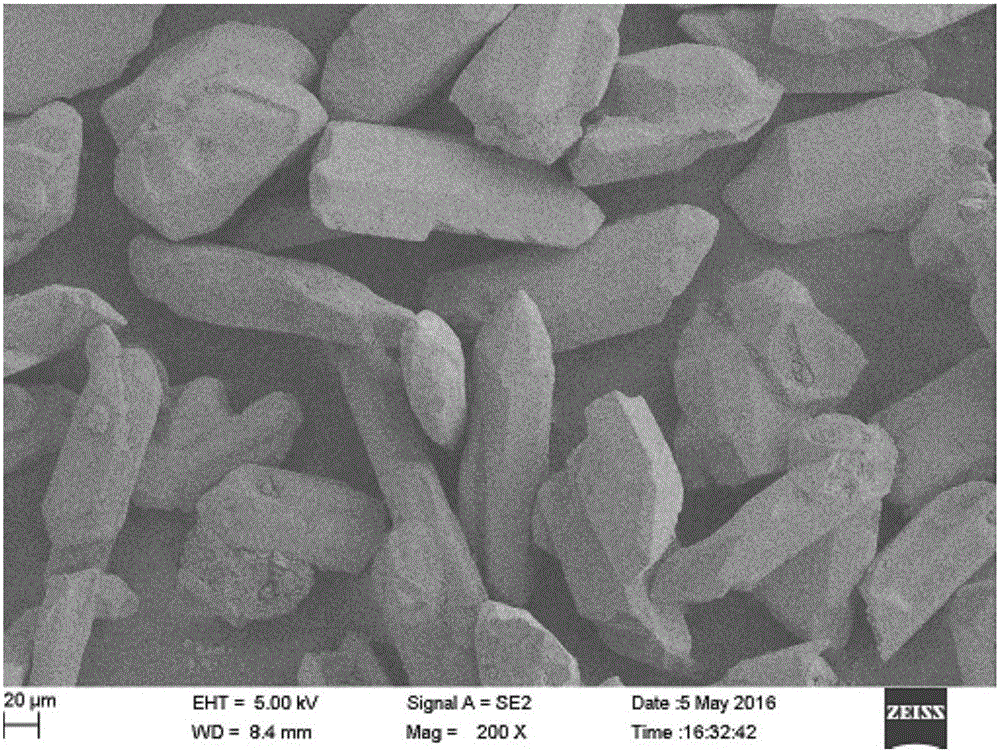
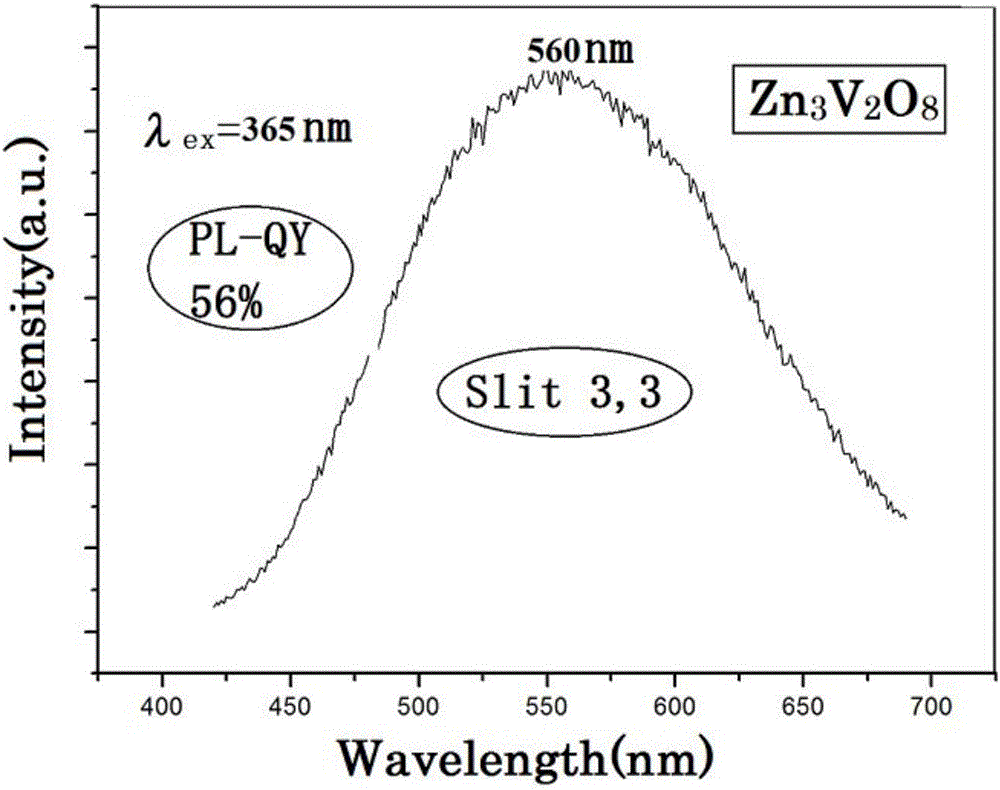
Image
Examples
Embodiment 1
[0014] Preparation of Zn 3 V 2 o 8 , the specific method is: take ZnO, V 2 o 5 and ZnCl 2 , the three are mixed and ground in turn according to the molar ratio of 3:1:2, and then reacted at 600°C for 4 hours, and the product is washed and dried to obtain pure Zn 3 V 2 o 8 .
Embodiment 2
[0016] Preparation of Zn 3 V 2 o 8 , the specific method is: take ZnO, V 2 o 5 and ZnCl 2 , the three were mixed and ground in turn according to the molar ratio of 3:1:2.5, and then reacted at 600°C for 4 hours, and the product was washed and dried to obtain pure Zn 3 V 2 o 8 .
Embodiment 3
[0018] Preparation (Zn 0.9 Eu 0.1 ) 3 V 2 o 8 , the specific method is: take ZnO, V 2 o 5 , ZnCl 2 and Eu 2 o 3 , according to the molar weight of ZnO: the molar weight of Eu: V 2 o 5 Molar mass: ZnCl 2 The molar weight is equal to 2.7:0.3:1:2, mixed and ground evenly, then reacted at 600°C for 4 hours, and the product was washed and dried to obtain pure (Zn 0.9 Eu 0.1 ) 3 V 2 o 8 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap