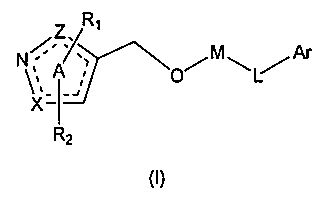
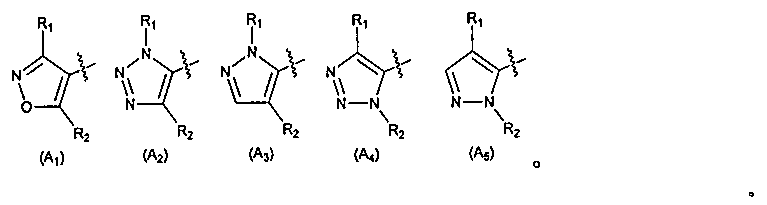
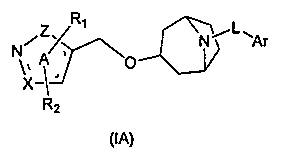
Heterocyclic farnesoid X receptor modifier
A technology of derivatives and compounds, applied in the field of isotope derivatives, can solve the problem of no non-steroidal FXR agonists
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0126] Compound preparation:
[0127] Intermediate 1:
[0128]
[0129]
[0130] Step 1: Under ice-bath condition, in the water (16.5mL) solution of hydroxylamine hydrochloride (22.5g, 362mmol), add dropwise sodium hydroxide aqueous solution (25g, 629mmol, be dissolved in 105mL water), stir under ice-bath for 30 minutes, will The above solution was added to a solution of 2,6-dichlorobenzaldehyde in ethanol (250 mL), and the reaction system was heated to 90° C. and stirred overnight. Cool to room temperature, concentrate under reduced pressure to obtain a crude product, recrystallize the crude product with ethanol / water (1:10), filter, and dry to obtain (E)-2,6-dichlorobenzaldehyde oxime (1-2) (50g, yield Yield 92%) is a white solid.
[0131] Step 2: Slowly add N-chlorosuccinimide (36 g , 268mmol, solution in 140mL of N,N-dimethylformamide), the dropwise addition was completed, the reaction system was stirred at 40°C for 1 hour, then diluted with ethyl acetate, the org...
Embodiment 1
[0146]
[0147] Step 1: Compound I-1-1
[0148] Methyl p-benzoate (11.7 mg, 0.065 mmol), HATU (25 mg, 0.065 mmol) and compound 2-2 (32 mg, 0.065 mmol) were dissolved in N,N-dimethylformamide (1 mL), in Add N,N-diisopropylethylamine (21mg, 0.16mmol) while cooling in an ice bath, react at room temperature and stir overnight, add water (2mL) to quench the reaction, and extract the aqueous phase with ethyl acetate (3mL×3), The organic phases were combined, washed successively with saturated ammonium chloride, water, saturated sodium bicarbonate and saturated brine, dried over anhydrous sodium sulfate, filtered and concentrated and purified with a thin-layer silica gel plate (2% methanol in dichloromethane solution). Compound I-1-1 (16 mg, yield: 44%) was an oily product.
[0149] m / z: [M+H] + 555.1;
[0150] Step 2: Compound I-1A
[0151] Compound I-1-1 (16 mg, 0.029 mmol) was dissolved in methanol (2 mL), lithium hydroxide (3 mg, 0.059 mmol) was dissolved in 1 mL of water ...
Embodiment 2
[0160]
[0161] Step 1: Compound I-2-1
[0162] Dissolve Intermediate 2-2 (32mg, 0.065mmol), triethylamine (16mg, 016mmol), 3-ethoxycarbonylphenylisocyanate (12.4mg, 0.065mmol) in dichloromethane (1mL), and stir at room temperature for 1 After 2 hours, the organic solvent was spin-dried, and the obtained crude product was purified by a thin-layer silica gel plate (2% methanol in dichloromethane solution) to obtain compound I-2-1 (30 mg, yield: 79%) as a pale yellow oil.
[0163] m / z: [M+H] + 583.2;
[0164] Step 2: Compound I-2A
[0165] Compound I-2-1 (30 mg, 0.05 mmol) was dissolved in methanol (2 mL), lithium hydroxide (11 mg, 0.25 mmol) was dissolved in 1 mL of water and added dropwise to the above solution, stirred at room temperature overnight, and the methanol was distilled off under reduced pressure , the aqueous phase was adjusted to a pH value approximately equal to 3 with 2N hydrochloric acid diluent, extracted with ethyl acetate (3mL×3), the organic phase was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com