Heat clearing fructus gardenia and radix ophiopogonis tablets and preparing method thereof
A technology for clearing fire gardenia oatmeal and gardenia, which is applied to separation methods, chemical instruments and methods, pharmaceutical formulations, etc., can solve the problems of low content of active ingredients, large patient dosage, inconvenient taking, etc., and achieves stable and suitable active ingredients. The effect is strong and the active ingredients are not destroyed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
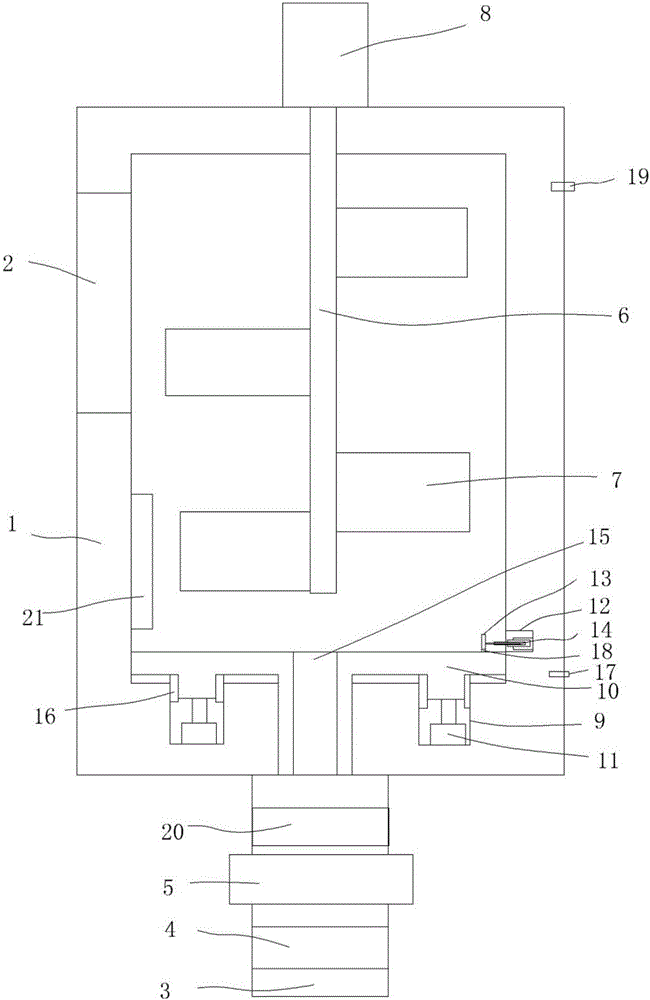
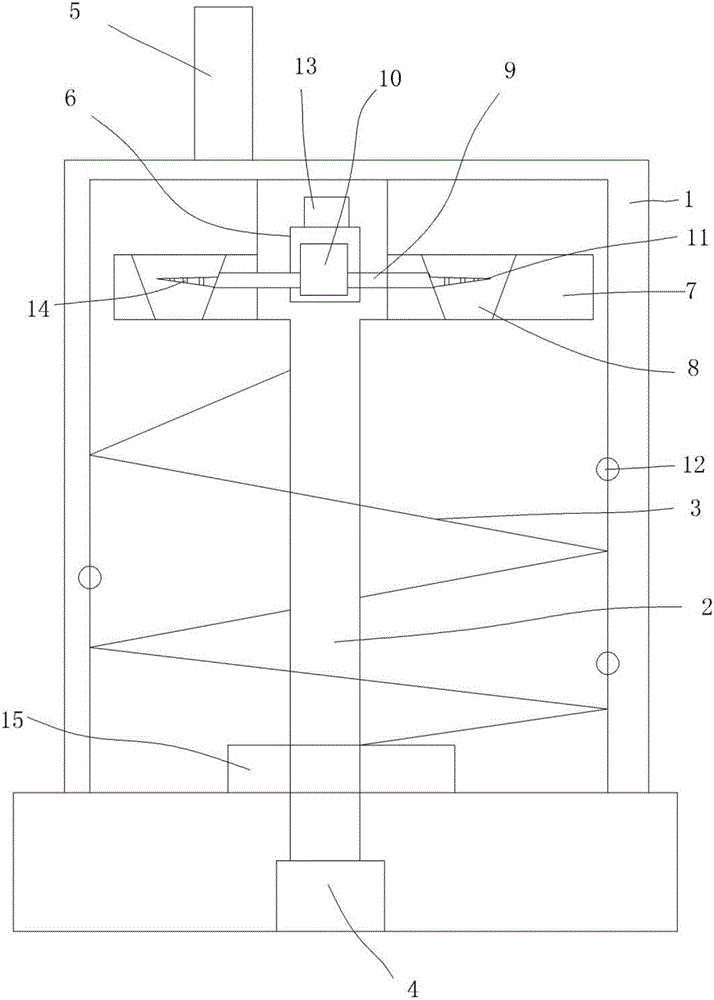
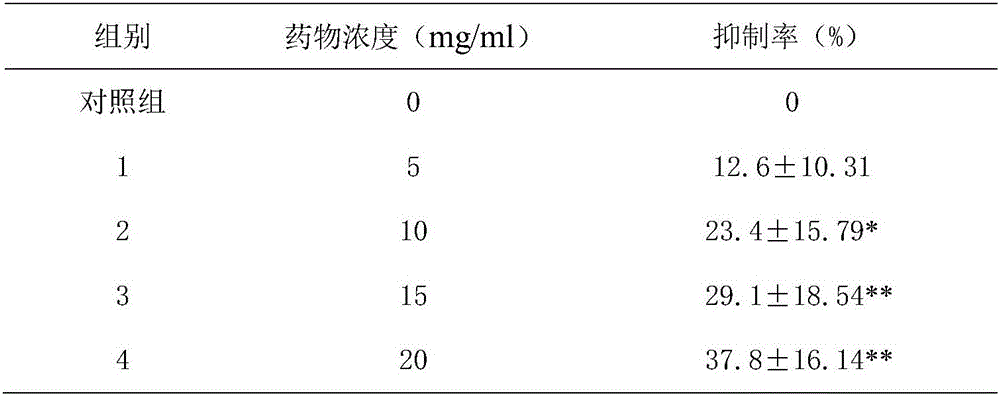
Image
Examples
Embodiment 1
[0021] Take 800g of Andrographis paniculata, 100g of gardenia, 100g of Radix Ophiopogon japonicus, the above three flavors, add water 10 times the weight of the medicinal materials, soak for 0.5h, decoct for 1 hour, filter, add the dregs of the medicine, add water 8 times the weight of the medicinal materials, and decoct for 1 hour, Filter out the medicinal liquid, combine two decoction liquids, concentrate to 60°C with a relative density of 1.05, add ethanol so that the volume percentage of alcohol content reaches 75%, stir, stand still, filter, and when the filtrate is concentrated to 65°C, the relative density is 1.25, and recover ethanol to obtain a concentrated solution, use supercritical extraction to obtain a supercritical extract, continue to concentrate, dry the concentrated solution, granulate, and compress into tablets to obtain Qinghuo Zhimai Tablets, 0.5 grams per tablet.
[0022] The preparation method of the Qinghuo Zhimai Tablets, the supercritical extraction me...
Embodiment 2
[0032] Take 800g of Andrographis paniculata, 100g of gardenia, 100g of Radix Ophiopogon japonicus, the above three flavors, add water 10 times the weight of the medicinal materials, soak for 0.5h, decoct for 1 hour, filter, add the dregs of the medicine, add water 8 times the weight of the medicinal materials, and decoct for 1 hour, Filter out the medicinal liquid, combine two decoction liquids, concentrate to 60°C with a relative density of 1.05, add ethanol so that the volume percentage of alcohol content reaches 75%, stir, stand still, filter, and when the filtrate is concentrated to 65°C, the relative density is 1.25, and recover ethanol to obtain a concentrated solution, use supercritical extraction to obtain a supercritical extract, continue to concentrate, dry the concentrated solution, granulate, and compress into tablets to obtain Qinghuo Zhimai Tablets, 0.5 grams per tablet.
[0033] The preparation method of the Qinghuo Zhimai Tablets, the supercritical extraction me...
Embodiment 3
[0036] Take 800g of Andrographis paniculata, 100g of gardenia, 100g of Radix Ophiopogon japonicus, the above three flavors, add water 10 times the weight of the medicinal materials, soak for 0.5h, decoct for 1 hour, filter, add the dregs of the medicine, add water 8 times the weight of the medicinal materials, and decoct for 1 hour, Filter out the medicinal liquid, combine two decoction liquids, concentrate to 60°C with a relative density of 1.05, add ethanol so that the volume percentage of alcohol content reaches 75%, stir, stand still, filter, and when the filtrate is concentrated to 65°C, the relative density is 1.25, and recover ethanol to obtain a concentrated solution, use supercritical extraction to obtain a supercritical extract, continue to concentrate, dry the concentrated solution, granulate, and compress into tablets to obtain Qinghuo Zhimai Tablets, 0.5 grams per tablet.
[0037] The preparation method of the Qinghuo Zhimai Tablets, the supercritical extraction me...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com