High efficient dechlorination composite material and preparation method thereof
A composite material, dechlorination technology, applied in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, water/sludge/sewage treatment, etc. Low cost, avoid agglomeration, increase the effect of specific area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
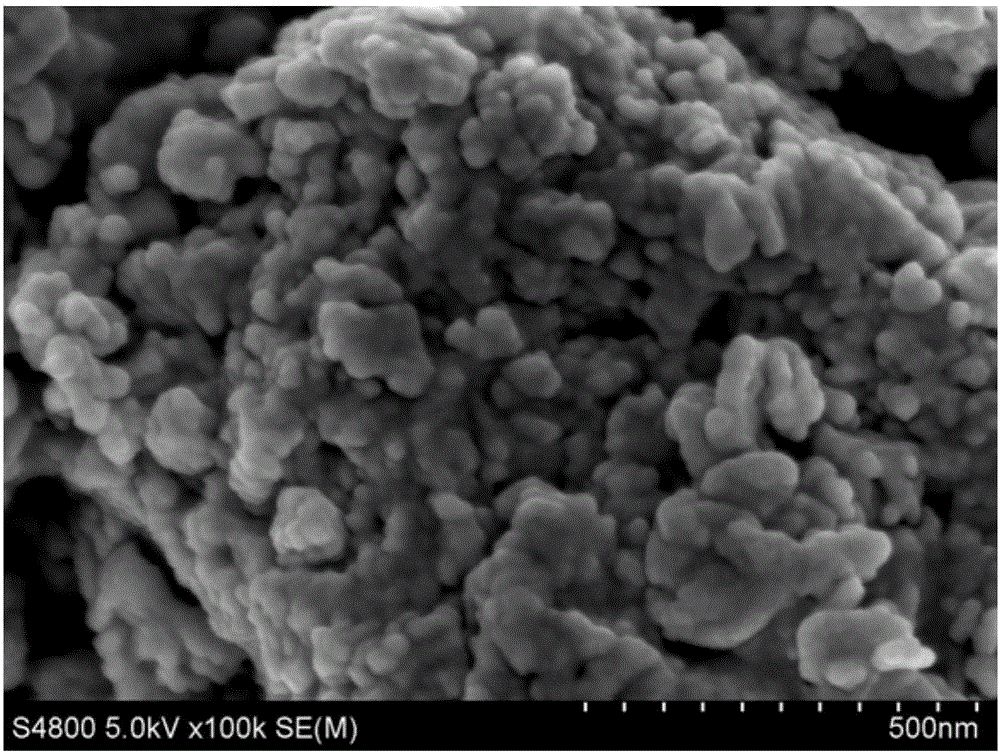
Image
Examples
Embodiment 1
[0026] Example 1: Preparation of starch-loaded iron-nickel bimetallic nanoparticles dechlorination composite material
[0027] Specific steps are as follows:
[0028] (1) Weigh 1.39g FeSO 4 ·7H 2 O and 2.256g of starch were dissolved in 100mL of anaerobic water, put into a three-necked flask, and passed through N 2 Stir for 15 minutes;
[0029] (2) Dispense 100mL of 0.8mol / L NaBH with a separatory funnel 4 The solution was slowly added dropwise to the Fe 2+ In the solution, a large amount of gas is generated during the dropwise addition reaction, and the N 2 , stirred for 15min, the generated H 2 Drive away in time; the theoretically obtained mass of zero-valent nano-Fe is 0.28g;
[0030] (3) Observe the reaction until no bubbles are generated, and continue to feed N 2 , then add 0.237g of NiCl 2 ·6H 2 0 to the three-necked flask, replace 20% Fe, react 15min to stop the reaction, theoretically obtain the nano-Fe / Ni bimetal containing 0.224g Fe, 0.058g Ni, and the the...
Embodiment 2
[0032] Example 2: Dechlorination experiment of starch-loaded iron-nickel bimetallic nanoparticles dechlorinated composite material to degrade trichlorethylene
[0033]The specific experimental steps are as follows:
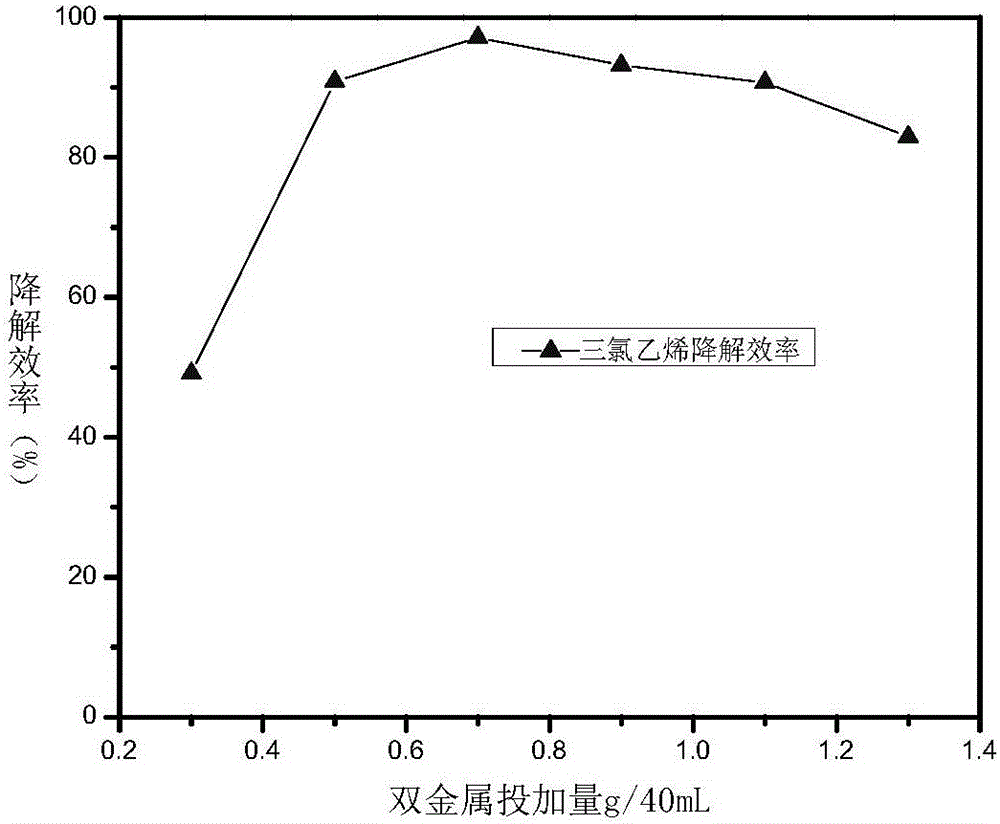
[0034] Weigh respectively the starch-loaded iron-nickel bimetallic nanoparticles prepared in Example 1 0.3g, 0.5g, 0.7g, 0.9g, 1.1g, 1.3g and put into 6 bottles of trichlorethylene solution (concentration is 73ppm) that 40mL is housed respectively Serum bottles, numbered 1-6, were filled with 40 mL of trichlorethylene solution (concentration: 73 ppm) without adding the dechlorination composite material as a blank control. Place room temperature stirring (650rpm) to test the dechlorination degradation effect after reacting for 10h, such as figure 2 As shown, except No. 1, the degradation efficiency of trichlorethylene in all samples is above 80%, the optimal dosage is 0.7g, and its degradation efficiency of trichlorethylene can reach 97.2% within 10 hours.
Embodiment 3
[0035] Example 3: Dechlorination experiment of starch-loaded iron-nickel bimetallic nanoparticles dechlorination composite material to degrade chloroform
[0036] The specific experimental steps are as follows:
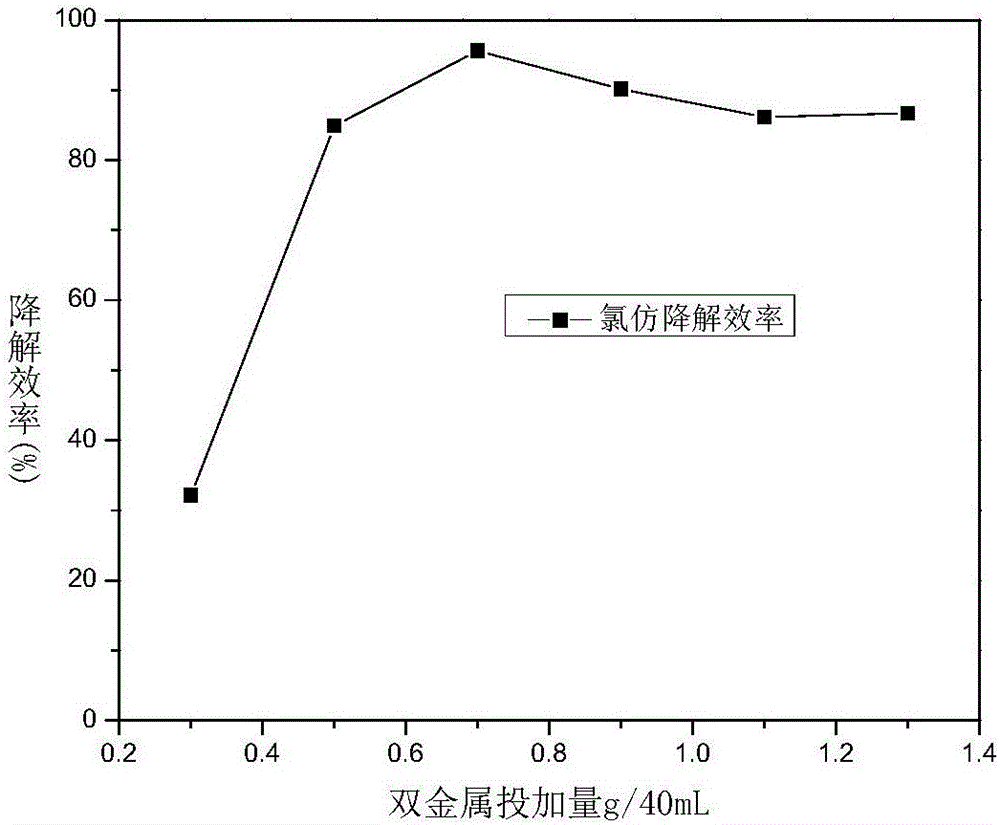
[0037] Take by weighing respectively the starch-loaded iron-nickel bimetallic nanoparticles 0.3g, 0.5g, 0.7g, 0.9g, 1.1g, and 1.3g prepared in Example 1 and put into 6 bottles of chloroform solution (concentration is 75ppm) serum bottles that are equipped with 40mL Among them, No. 1-6, 40 mL of chloroform solution (concentration of 75 ppm) was filled with no dechlorination composite material as a blank control. Place room temperature stirring (650rpm) to test the dechlorination degradation effect after reacting for 10h, such as image 3 As shown, except No. 1, the degradation efficiency of chloroform in all samples is above 80%, the optimal dosage is 0.7g, and its degradation efficiency to chloroform can reach 95.6% within 10 hours.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com