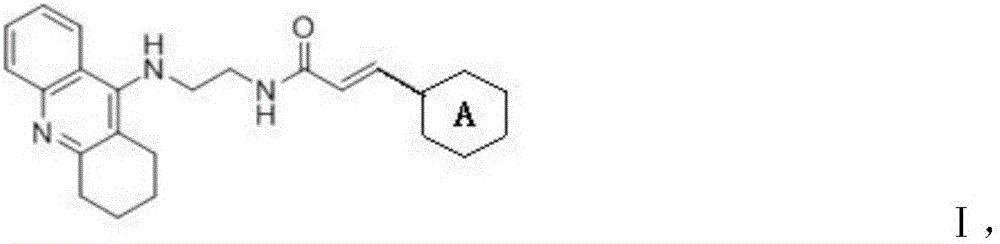
Compounds having anti-Alzheimer's disease effect, and preparation method and application thereof
A technology for compounds and nitrogen compounds, applied in the field of compounds, can solve the problems of lack of effective binding of PAS sites, limited anti-AD activity, etc., and achieve the effects of reasonable process design, high purity and few by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
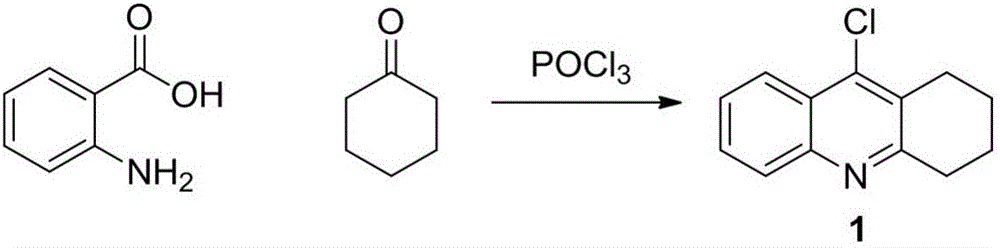
[0029] Synthesis of 9-chloro-1,2,3,4 tetrahydroacridine (compound 1)
[0030]
[0031] Add anthranilic acid (5g, 36.2mmol) and cyclohexanone (3.6g, 36.2mmol) into a di[neck flask, add phosphorus oxychloride (15ml in total) dropwise in an ice bath, and stir for 5 minutes in an ice bath , heated in an oil bath to 115°C. The reaction solution was refluxed for about 5 hours and cooled to room temperature. Dilute with 80ml of ice DCM, add 40ml of water to the ice bath, and adjust the pH to 9. The organic layer was separated, extracted three times with DCM, washed three times with brine, and dried over anhydrous sodium sulfate. Spin dry, and separate by column chromatography (developing solvent: PE / EA=8 / 1, v / v). A tan solid was finally obtained with a yield of 86%.
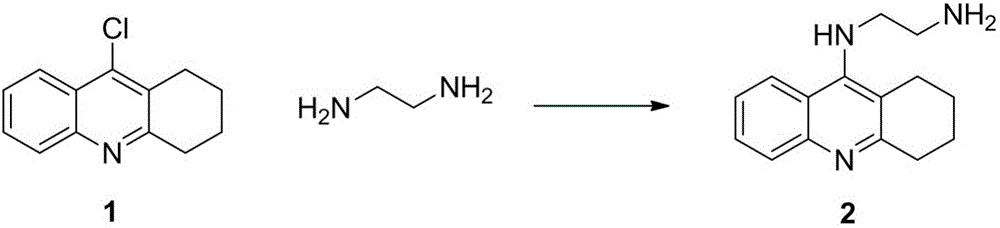
[0032] Synthesis of 9-(β-aminoethylenediamine)-1,2,3,4-tetrahydroacridine (compound 2)
[0033]
[0034] Add ethylenediamine (1.44g, 6mmol) and a catalytic amount of NaI into a 100ml two-necked flask, add 22m...
Embodiment 2
[0036] Example 2 (E)-N-(2-((9-(1,2,3,4-tetrahydroacridinyl)) amino) ethyl) synthesis of cinnamic acid amides (3)
[0037]
[0038] Trans-cinnamic acid (0.147g, 0.1mmol), PyBOP (0.56g, 1.08mmol), DIPEA (0.21g, 1.66mmol) were dissolved in 5ml of DCM and stirred at room temperature for 1 hour. Then compound 2 (0.2 g, 0.83 mmol) of Example 1 was added into the reaction flask, and stirred at room temperature overnight. The reaction was quenched with 15 ml of water, and the organic layer was separated and dried over anhydrous sodium sulfate. Spin to dry, and separate by column chromatography (developer: DCM / MeOH=30 / 1, v / v, add 1ml triethylamine per 1000ml developer). 0.21 g of the product was obtained with a yield of 68%. 1 H-NMR (CDCl 3 ):δ8.04-8.01(m,2H,arom),7.64-7.56(m,2H,arom),7.51-7.45(m,3H,arom),7.31-7.25(m,3H,arom,COCH=C H ), 6.34 (d, J=15Hz, 1H, COC H =CH),3.40-3.36(m,2H,NHC H 2 ),3.04(br,2H,C H 2 NHCO),2.87(m,2H,C4-H 2 ),2.67(m,2H,C1-H 2 ),1.81-1.74(m,4H,C2-H...
Embodiment 3
[0039] Example 3 (E)-3-(o-tolyl)-N-(2-((9-(1,2,3,4-tetrahydroacridinyl))amino)ethyl)acrylic acid (compound 4) Synthesis
[0040]
[0041] Referring to the synthesis method in Example 2, compound 4 was synthesized using 2-methyltrans-cinnamic acid (0.16 g, 0.1 mmol) as a raw material. Yield 80%. 1 H-NMR (CDCl 3 ):δ8.04-8.01(m,2H,arom),7.64-7.56(m,2H,arom),7.51-7.45(m,2H,arom),7.31-7.25(m,3H,arom,COCH=C H ), 6.34 (d, J=15Hz, 1H, COC H =CH),3.40-3.36(m,2H,NHC H 2 ),3.04(br,2H,C H 2 NHCO),2.87(m,2H,C4-H 2 ),2.67(m,2H,C1-H 2 ),2.22(s,3H,C H 3 ),1.81-1.74(m,4H,C2-H 2 ,C3-H 2 ).HRMS(ESI):calcd for C 25 h 27 N 3 O[M+H] + 385.50, found 385.5004.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com