A kind of allergy test method for Acanthopanax injection
A technology of Acanthopanax injection and inspection method, which is applied in the direction of microbial measurement/inspection, biochemical equipment and methods, instruments, etc., and can solve the problems of inaccurate reflection of actual sensitization conditions, long time-consuming, large differences, etc. , to achieve convenient and intuitive evaluation and judgment, reduce the risk of occurrence, and improve the effect of comparison standards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1 A kind of anaphylactoid test method of Acanthopanax injection
[0047] The steps are:
[0048] (1), sample pretreatment: put the Acanthopanax injection in a 40°C water bath for 30 minutes, and immediately centrifuge it at 6000rpm for 60s, take the supernatant, and dilute the supernatant 10 times with phenol red-free serum-free MEM medium, Obtain the liquid to be tested;
[0049] (2), prepare detection cell: use the MEM culture that contains 1% penicillin-streptomycin double antibiotics and 10% heat-inactivated fetal bovine serum to cultivate RBL-2H3 cells based on 37 ℃, 5% CO2 cell incubator, when When the RBL-2H3 cells grow to cover 90% of the bottom area of the culture flask, they are subcultured at a ratio of 1:5, once every other day, and then subcultured 3 times to obtain normally growing RBL-2H3 cells, which are the test cells;
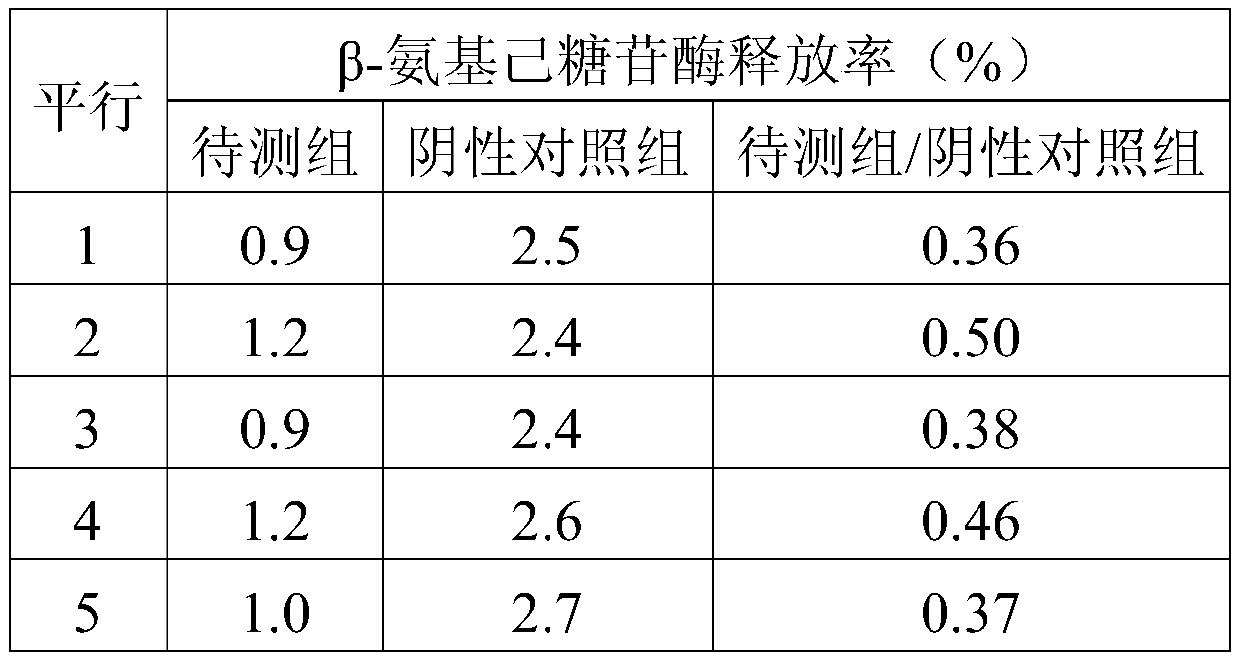
[0050] (3), β-hexosaminidase release rate detection: according to 2 × 10 5 Inoculation density per well The test cells...
Embodiment 2
[0058] Embodiment 2 A kind of anaphylactoid test method of Acanthopanax injection
[0059] The steps are:
[0060] (1), sample pretreatment: put Acanthopanax injection in a water bath at 45°C for 25 minutes, then centrifuge at 7000rpm for 50s immediately, take the supernatant, and dilute the supernatant 11 times with phenol red-free serum-free MEM medium, Obtain the solution to be tested.
[0061] (2), prepare detection cell: use the MEM culture that contains 1% penicillin-streptomycin double antibiotics and 10% heat-inactivated fetal bovine serum to cultivate RBL-2H3 cells based on 37 ℃, 5% CO2 cell incubator, when When the RBL-2H3 cells grow to cover 90% of the bottom area of the culture flask, they should be subcultured at a ratio of 1:6, once every other day, and then twice, to obtain normal growing RBL-2H3 cells, which are the test cells;
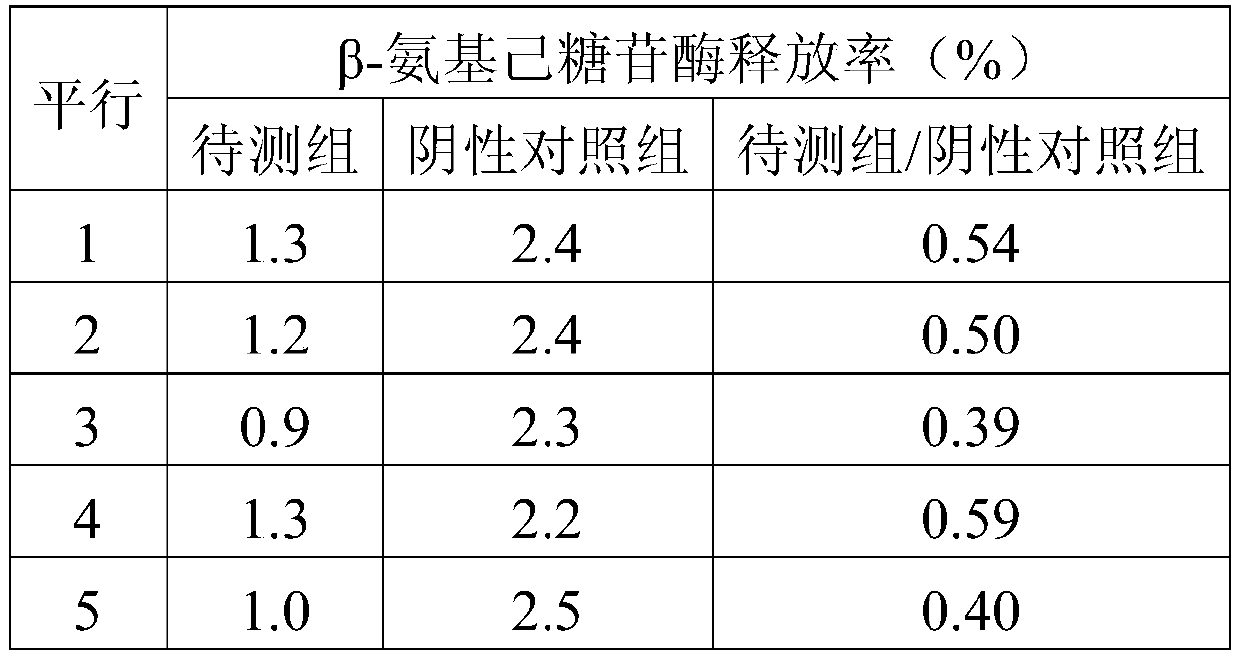
[0062] (3), β-hexosaminidase release rate detection: according to 5 × 10 5 Inoculation density per well The test cells obtained ...
Embodiment 3
[0070] Embodiment 3 A kind of anaphylactoid test method of Acanthopanax injection
[0071] The steps are:
[0072] (1), sample pretreatment: put Acanthopanax injection in a water bath at 50°C for 20 minutes, then centrifuge it at 8000rpm for 40s immediately, take the supernatant, and dilute the supernatant 12 times with phenol red-free serum-free MEM medium, Obtain the solution to be tested.
[0073] (2), prepare detection cell: use the MEM culture that contains 1% penicillin-streptomycin double antibiotics and 10% heat-inactivated fetal bovine serum to cultivate RBL-2H3 cells based on 37 ℃, 5% CO2 cell incubator, when When the RBL-2H3 cells grow to cover 90% of the bottom area of the culture flask, they should be subcultured at a ratio of 1:7, once every other day, and then twice, to obtain normal growing RBL-2H3 cells, which are the test cells;
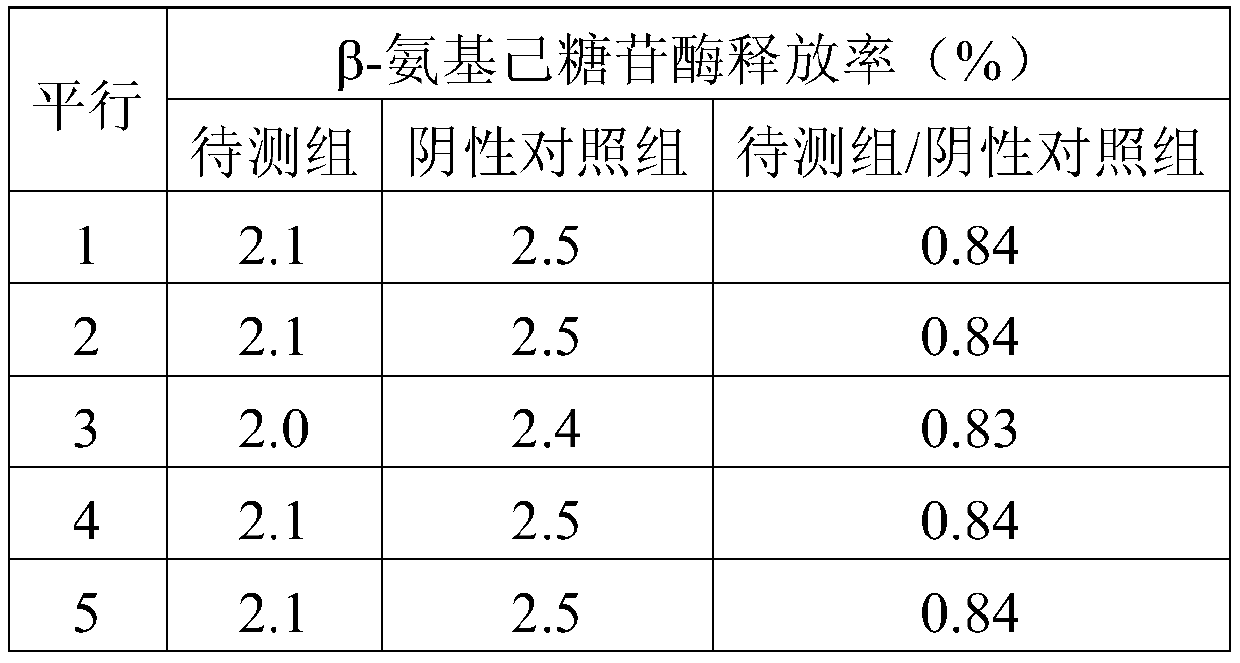
[0074] (3), β-hexosaminidase release rate detection: according to 3 × 10 5 Inoculation density per well The test cells obtained...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com